Introduction To Stoichiometry Worksheet Answers Introduction You might use stoichiometry skills to double a cookie recipe Image credit Chocolate Chip Cookies by Kimberley Vardeman on Wikimedia Commons CC BY 2 0 A balanced chemical equation is analogous to a recipe for chocolate chip cookies It shows what reactants the ingredients combine to form what products the cookies
An introduction to Stoichiometry You have spent a lot of time studying the various types of reactions that can occur in chemistry You have also become experts in balancing chemical equations In this activity you will be introduced to simple stoichiometry Download Introduction to Stoichiometry Worksheet with Answers and more Chemistry Exercises in PDF only on Docsity Chapter 9 Stoichiometry Stoichiometry A Relating the mass of the is Dtoarchiomatny 1 Stoichiometry follows the Law of Conservation of Y G Ss 2
Introduction To Stoichiometry Worksheet Answers
Introduction To Stoichiometry Worksheet Answers
http://www.worksheeto.com/postpic/2012/06/stoichiometry-worksheet-answers_670813.jpg
Basic Stoichiometry Phet Lab Answers 2 This Basic Stoichiometry
https://www.chemedx.org/sites/www.chemedx.org/files/stoichlab2.png

Stoichiometry Limiting Reagent Worksheet Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/limiting-reagent-worksheet-1-4.png
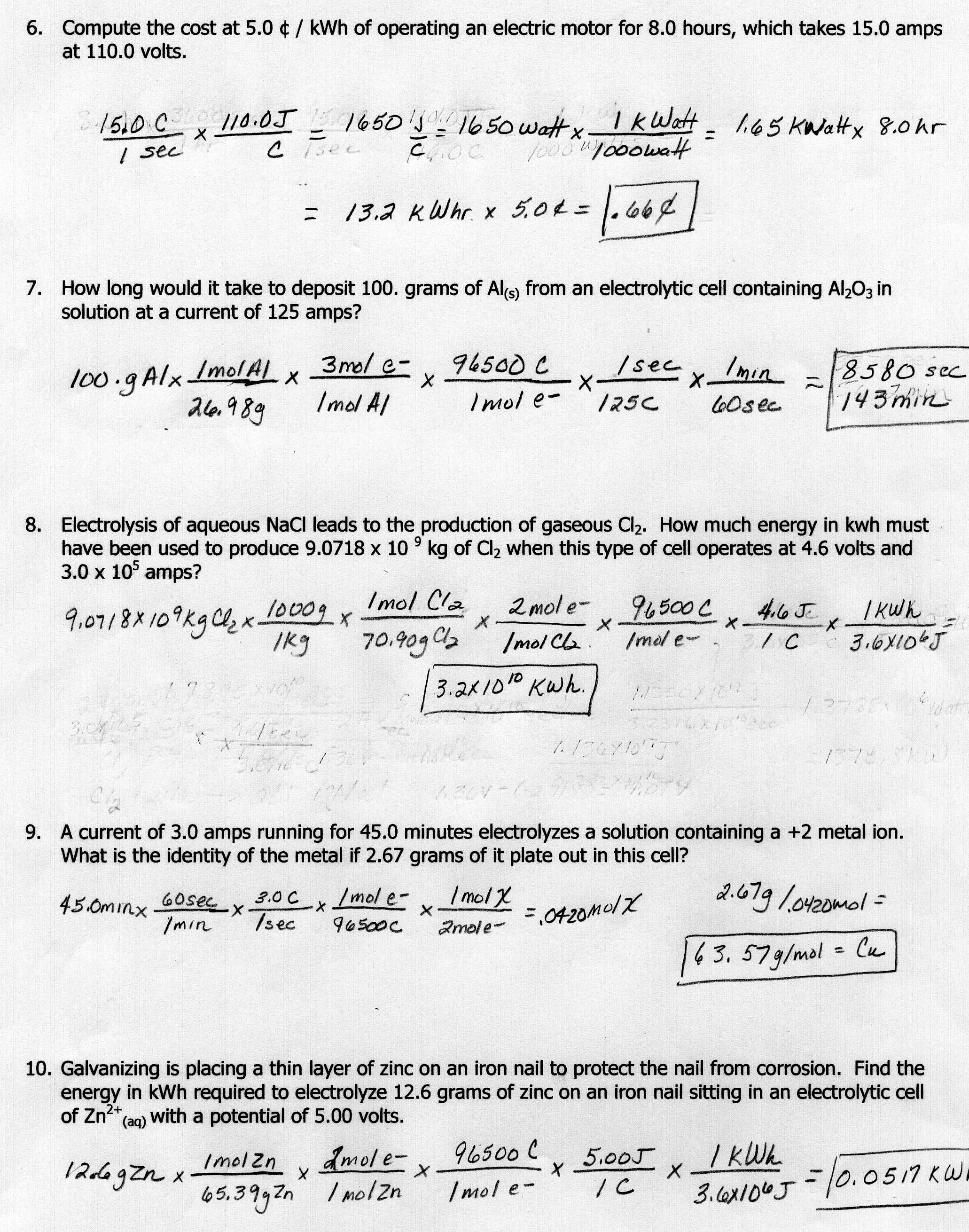
Q4 Given the following reaction H2SO4 Na2CO3 Na2SO4 H2O CO2 Calculate the molarity of the H2SO4 solution if it takes 40 0 mL of H2SO4 to neutralize 46 7 mL of a 0 364 M Na2CO3 solution Balance the equation Apply the Law of Conservation of Mass a relation stating that in a chemical reaction the mass of the products equals the mass of the reactants to get the same number of atoms of every element on each side of the equation Tip Start by balancing an element that appears in only one reactant and product
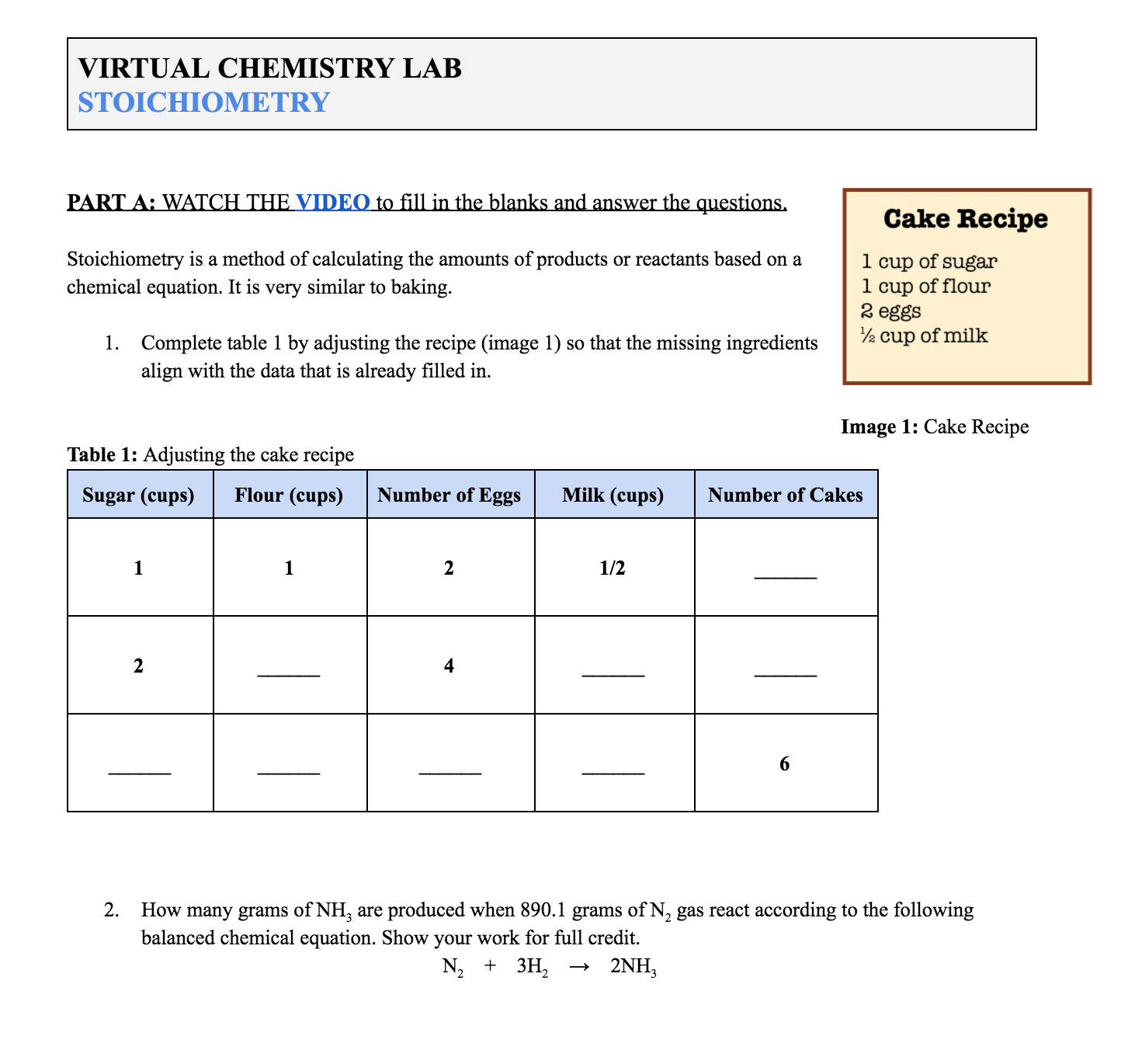
Section 1 Introducing Stoichiometry The activity begins with a short video video 1 that introduces stoichiometry with a simple baking analogy After answering related questions on a worksheet see supporting information the video teaches students to perform stoichiometric calculations and then applies them in a simple experiment Stoichiometry WorkSheet 1 Worked Solutions Answer the following questions on your own paper Show all work Circle the final answer giving units and the correct number of significant figures 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H3PO4
More picture related to Introduction To Stoichiometry Worksheet Answers

Stoichiometry Worksheet 1 Mass Mass
https://images.sampletemplates.com/wp-content/uploads/2016/11/17162322/Mass-to-Mass-Stoichiometry-Worksheet.jpg
Stoichiometry Practice 2 Worksheet Answers Printable Word Searches
https://i2.wp.com/lh3.googleusercontent.com/proxy/YLfQO2Ktop3x54BhOh9tk13b7HDzDXRDMDkZ58yQ1ElhX3qHqEpxKSglUvtaUfhfmNx-mpIWj4NApaAlHN8bvPRDERlmJSpAcXXzhst3hzZG-KDFUVYdCrSlipnNYpAJ=w1200-h630-p-k-no-nu

8 Best Images Of Organic Chemistry Review Worksheets Chemistry
http://www.worksheeto.com/postpic/2012/01/chemistry-stoichiometry-worksheet-answer-key_682800.jpg
To do this start by dividing the smallest number of moles into each of the numbers of moles of elements i e set the smallest number to 1 This may yield integers or it may yield decimal results that correspond closely to rational fractions For example 1 25 2 75 1 2 5 11 1 Fermentation is a complex chemical process of making wine by converting glucose into ethanol and carbon dioxide C6H12O6 s 2 C2H5OH l 2 CO2 g A Calculate the mass of ethanol produced if 500 0 grams of glucose reacts completely 500 0 g C6H12O6 1 mol C H 6 12
A How many grams of NaOH is produced from 1 20 x 102 grams of Na2O 154 8 g NaOH b How many grams of Na2O are required to produce 1 60 x 102 grams of NaOH 124 g Na2O 5 Given the following equation 8 Fe S8 8 FeS a What mass of iron is needed to react with 16 0 grams of sulfur 27 87 g Fe b Agenda Lab Introduction to stoichiometry HW 1 lab calculations 2 read p 237 240 Wednesday February 15 Agenda 1 Go over lab 2 worksheet 3 HW 1 read p 242 2 video notes Thursday February 16 Agenda 1 Stoichiometry worksheet 4 HW 1 p 262 36 37 and 40 2 worksheet 5 Answers to worksheet 6

Gas Stoichiometry Worksheet
https://s3.studylib.net/store/data/008203192_1-2464a83abc4de00c0bc35eab46d84f34-768x994.png

Intro To Stoichiometry Worksheets Answers
https://i.pinimg.com/originals/e7/99/0b/e7990b8ad502dabd90a5477f6445bb9e.png
Introduction To Stoichiometry Worksheet Answers - Matter Ch 1 Worksheet Matter Lab Classifying Matter with Hardware Lab Classification Rotation Lab Chemical Physical Changes option 1 Lab Chemical Physical Changes option 2 Activity Categorizing Matter Measurement Ch 2 Worksheet Using Measurements