Experiment 12 Single Displacement Reactions All single displacement reactions have the general form A BC B AC 6 1 6 1 A B C B A C Here A A is an element and BC B C is usually an aqueous ionic compound or an acid consisting of B B and C C aqueous ions A displaces B B in BC B C resulting in the formation of a new element B B and a new ionic compound or
There are 3 steps to solve this one Expert verified Share Share Step 1 THE SOLUTION IS Understanding Single Displacement Reactions In single displacement reactions a more r View the full answer Step 2 Unlock Step 3 Unlock Section 1 Purpose and Summary Carry out single displacement reactions involving a metals with salts of other metals and b metals with dilute acid Determine the order of activity of metals and hydrogen based on observations Note Single displacement reactions are sometimes called single replacement reactions
Experiment 12 Single Displacement Reactions
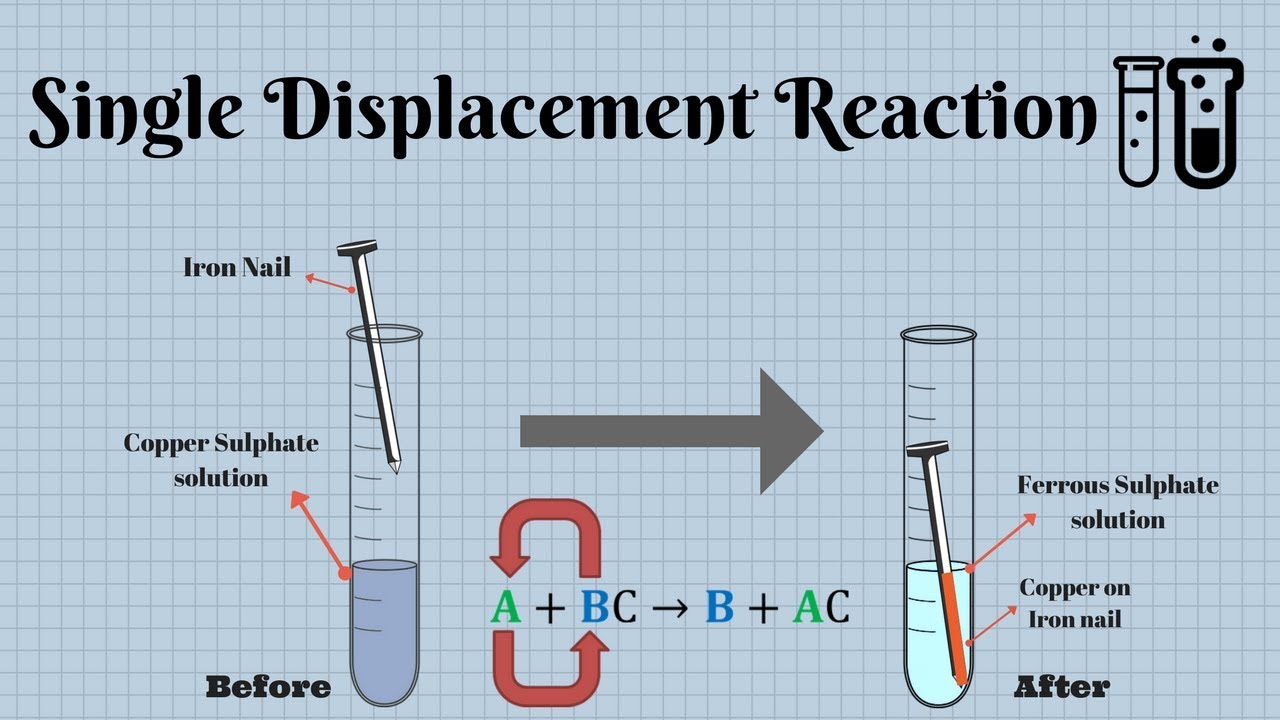
Experiment 12 Single Displacement Reactions
https://i.ytimg.com/vi/otq7f2BknF4/maxresdefault.jpg
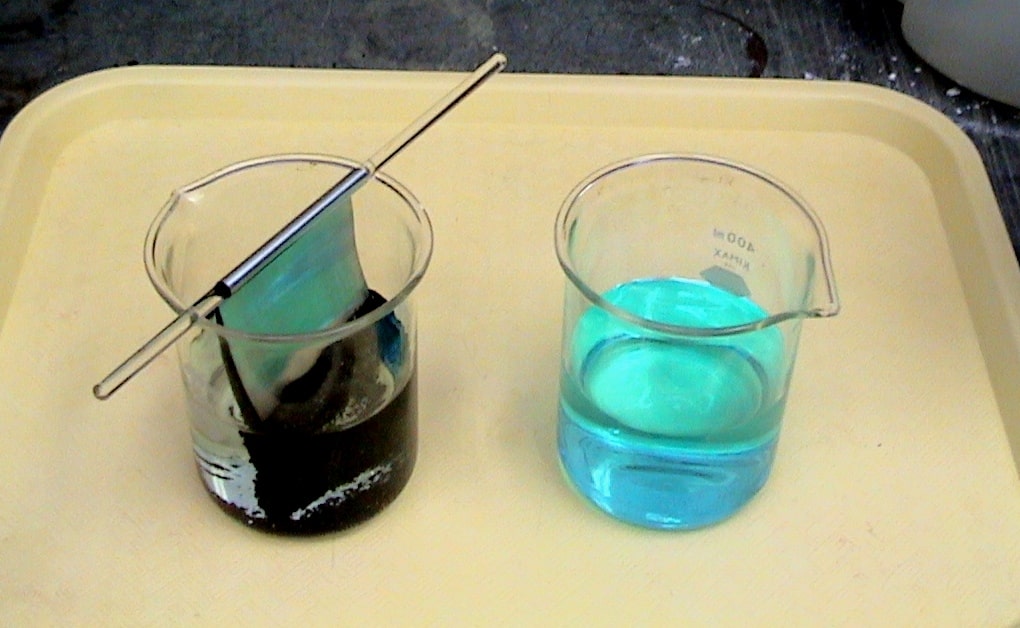
Single Displacement Reactions Lab Explained SchoolWorkHelper
https://schoolworkhelper.net/wp-content/uploads/2015/07/Zinc-Copper-Sulfate-reaction.jpeg
Solved SECTIONDATE INSTRUCTOR B A REPORT FOR EXPERIMENT 12 Chegg
https://media.cheggcdn.com/media/053/05311060-b2a0-4838-98dc-149db1cd4b23/image
A single replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products For example 7 4 1 2 HCl aq Zn s ZnCl 2 aq H 2 g is an example of a single replacement reaction The hydrogen atoms in HCl are replaced by Zn Home Science Single Displacement Reactions Lab Explained Purpose Two observe two different single displacement reactions Hypothesis When zinc is added to copper II sulfate a single displacement reaction will take place creating a solid copper and zinc sulfate
A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound A single displacement reaction is a chemical reaction where one reactant is exchanged for one ion of a second reactant It is also known as a single replacement reaction Single displacement reactions take the form A BC B AC Single Displacement Reaction Examples
More picture related to Experiment 12 Single Displacement Reactions
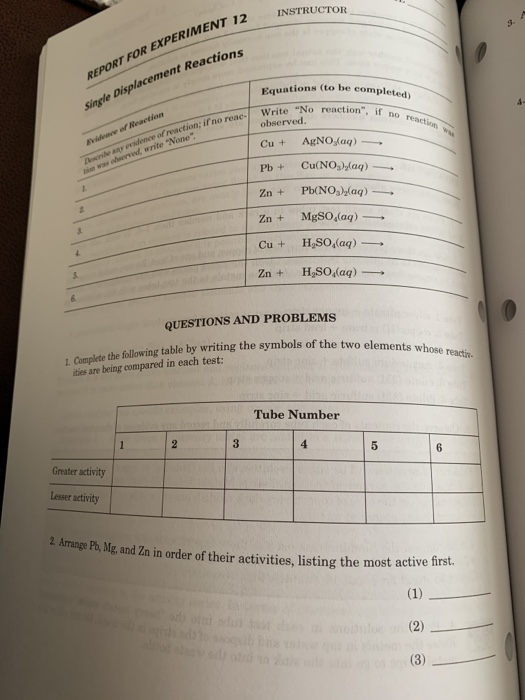
Solved REPORT FOR EXPERIMENT 12 Single Displacement Chegg
https://media.cheggcdn.com/media/a95/a956268e-4f46-4cdf-ab01-6ffcb0a1e1fa/image.png

Solved REPORT FOR EXPERIMENT 12 Single Displacement Chegg
https://media.cheggcdn.com/media/9d6/9d6d9fab-1402-47bb-9c64-3714f3f62a4d/image.png
Single Displacement Reactions Understanding With Examples
https://psiberg.com/wp-content/uploads/2023/03/Single-displacement-reactions.svg
This reaction can be classified as a single displacement reaction but it is also a redox reaction since the copper is gaining electrons from the zinc atoms to produce the copper atoms and zinc ions in solution Discussion The chemical reactivity of an element is related to its tendency to lose or gain electrons In theory it is possible to arrange nearly all the elements into a single series in order of their reactivities
PURPOSE Reactions of metals with acids and salt solutions Determine the activity of metals Write a balanced molecular equation complete ionic equation and net ionic equation for single replacement reaction Materials Example 9 1 9 1 Reaction Equation magnesium metal aqueous aluminum chloride Since Mg is more active than Al a single replacement reaction will occur The predicted products are aluminum metal and aqueous magnesium chloride 3 Mg 2 AlCl 3 2 Al 3 MgCl 2 A BC B AC Example 9 2 9 2
Solved SECTIONDATE INSTRUCTOR B A REPORT FOR EXPERIMENT 12 Chegg
https://media.cheggcdn.com/media/3cc/3cc1610e-731a-4188-9039-b4f27629bb08/image
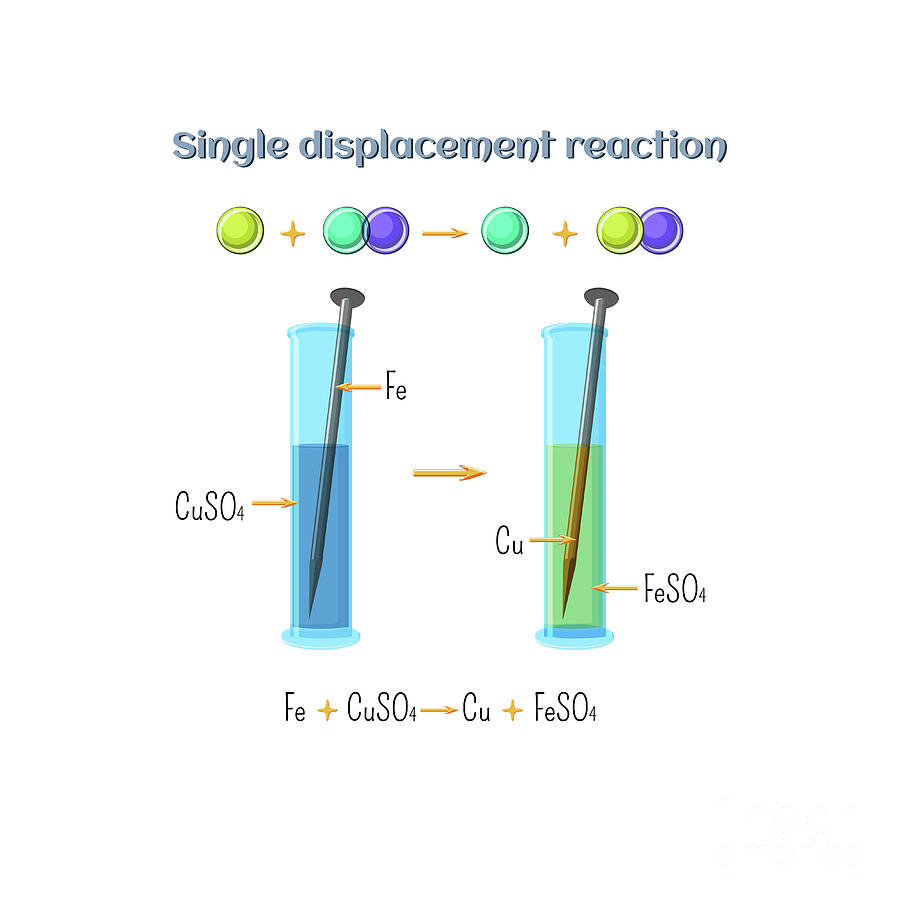
Single Displacement Reaction Photograph By Inna Bigun science Photo Library
https://images.fineartamerica.com/images/artworkimages/mediumlarge/2/single-displacement-reaction-inna-bigunscience-photo-library.jpg
Experiment 12 Single Displacement Reactions - Home Science Single Displacement Reactions Lab Explained Purpose Two observe two different single displacement reactions Hypothesis When zinc is added to copper II sulfate a single displacement reaction will take place creating a solid copper and zinc sulfate

