Which Of The Following Are Balanced Equations If a fractional coefficient has been used multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced Example 7 4 1 7 4 1 Combustion of Heptane
A balanced chemical equation shows the same number of each type of atom on both sides of the arrow Questions Tips Thanks Want to join the conversation Sort by Top Voted Gabrielle M 9 years ago I m working on Chemical Reactions Double and Single Replacement on FLVS Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems
Which Of The Following Are Balanced Equations

Which Of The Following Are Balanced Equations
https://i.ytimg.com/vi/BEP9O52w3SE/maxresdefault.jpg
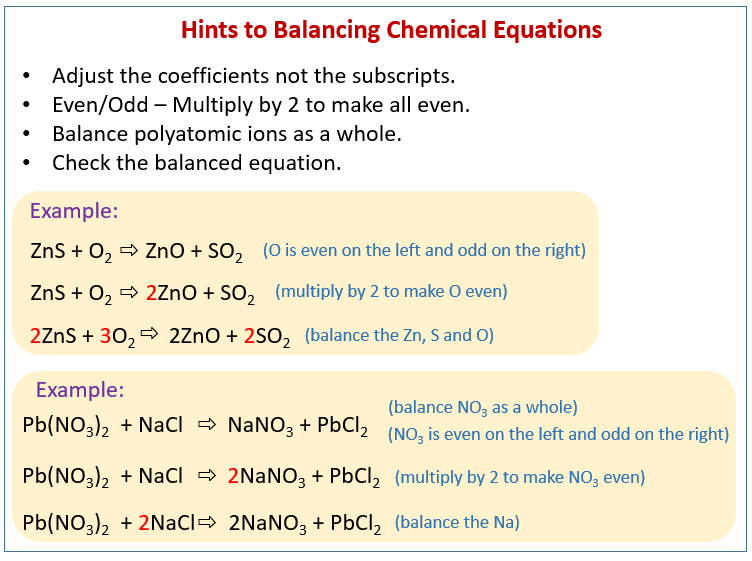
How To Balance Chemical Equations solutions Examples Videos
https://www.onlinemathlearning.com/image-files/xbalance-chemical-equations.png.pagespeed.ic.mtPzrx5_1V.png
Balancing Equations
http://www.sliderbase.com/images/referats/1120b/(11).PNG
Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the equation and only
To balance a redox equation using the half reaction method the equation is first divided into two half reactions one representing oxidation and one representing reduction The equations for the half reactions are then balanced for mass and charge and if necessary adjusted so that the number of electrons transferred in each equation is the same To balance a chemical equation enter an equation of a chemical reaction and press the Balance button The balanced equation will appear above Use uppercase for the first character in the element and lowercase for the second character Examples Fe Au Co Br C O N F Ionic charges are not yet supported and will be ignored
More picture related to Which Of The Following Are Balanced Equations
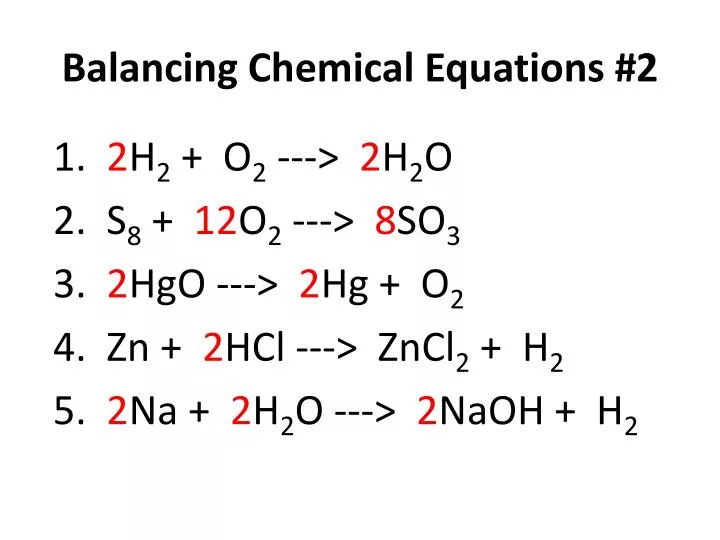
PPT Balancing Chemical Equations 2 PowerPoint Presentation Free
https://image2.slideserve.com/3871925/balancing-chemical-equations-2-n.jpg
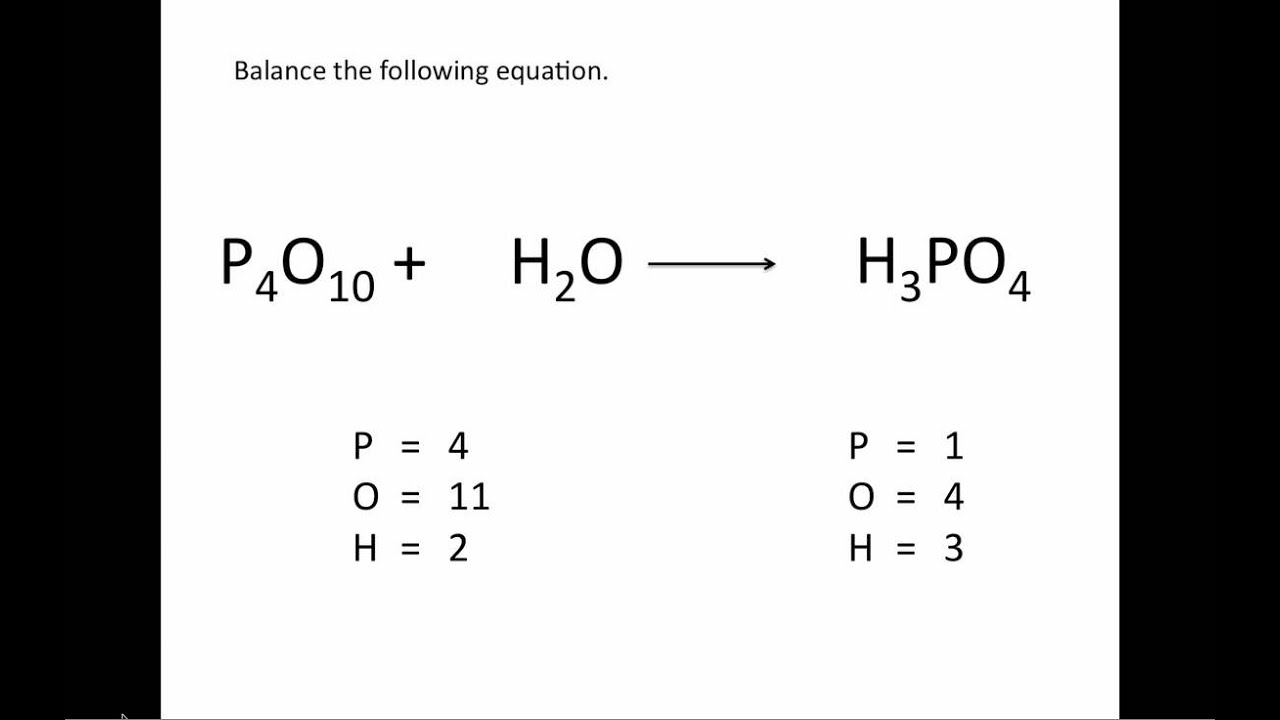
Balancing Chemical Equations UPDATED Chemistry Tutorial YouTube
https://i.ytimg.com/vi/HB6cG7bQew0/maxresdefault.jpg

How To Balance Chemical Equations 10 Steps with Pictures
http://www.wikihow.com/images/b/b8/Balance-Chemical-Equations-Step-10Bullet1-Version-2.jpg
A chemical equation describes what happens in a chemical reaction The equation identifies the reactants starting materials and products resulting substances the formulas of the participants the phases of the participants solid liquid gas the direction of the chemical reaction and the amount of each substance Chemical equations are balanced for mass and charge meaning the number Is the following chemical equation balanced 2Na F 2NaF Click the card to flip 1 35 Flashcards Learn Test Match Q Chat Created by mrs haley shipley Teacher For each of the following determine whether or not the equation is balanced Answer yes or no Students also viewed CHEM 285 Exam 3 33 terms elysedoyl Preview Amino Acids Trivia
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation We can write a chemical equation for the reaction of carbon with hydrogen gas to form methane CH4 CH 4 C s 2Catoms H2 g 2Hatoms CH4 g 1 Catom 4H atoms C s H 2 g CH 4 The Traditional Balancing Method The first step that must be followed while balancing chemical equations is to obtain the complete unbalanced equation In order to illustrate this method the combustion reaction between propane and oxygen is taken as an example Step 1
Balancing Chemical Equations Presentation Chemistry
http://www.sliderbase.com/images/referats/1120b/(8).PNG

How To Balance Chemical Equations YouTube
https://i.ytimg.com/vi/iUARzSxcKzk/maxresdefault.jpg
Which Of The Following Are Balanced Equations - Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the equation and only
.PNG)
.PNG)