Stoichiometry Packet Answer Key This substantial bundle contains everything you would need for a complete advanced chemistry course at least two sets of PowerPoints the Guided Practice Questions answer key to engage students during instruction an average of 4 6 review documents answer keys a list of key terms an 244 Products 475 00 521 00 Save 46 00 View Bundle
Question 10 Identify the energy graphs A and B as being for an endothermic or exothermic reaction A B Answer 4 7 Unit 4 Practice Problems is shared under a not declared license and was authored remixed and or curated by LibreTexts Stoichiometry is a collective term for the quantitative relationships between the masses Converting amounts of substances to moles and vice versa is the key to all stoichiometry problems whether the amounts are given in units of mass grams or kilograms weight pounds or tons or volume liters or gallons Answer 86 2 g
Stoichiometry Packet Answer Key

Stoichiometry Packet Answer Key
https://i.pinimg.com/originals/3d/f7/d3/3df7d3b6b0b0b9e87f1ebb771a4ed1cb.jpg

Unit 08 Stoichiometry Worksheet 1 With Answer Key Download
https://data.templateroller.com/pdf_docs_html/105/1051/105154/page_4_thumb_950.png

Tips On Using Stoichiometry Gizmo Answer Key In 2023 Athens Mutual
https://i.pinimg.com/originals/62/2b/e9/622be9f37eb7f34809faca3112dd016b.jpg
2 Add 20 ml of water to the calcium chloride 3 Using your pre lab data and the electronic balance measure the correct mass of sodium carbonate into a small beaker 4 Add 20 ml of water to the sodium carbonate 5 Mix the individual solutions with a stirring rod until all of the solid has dissolved Chapter 3 Stoichiometry Michigan State UniversityLearn the basic concepts and calculations of stoichiometry the quantitative study of chemical reactions in this introductory chemistry course from MSU Download the PDF lecture notes and practice with exercises and examples Explore how stoichiometry can be applied to real world problems such as environmental issues industrial processes
Is a unit of measurement in chemistry that represents the amount of a substance One mole of a substance is equal to Avogadro s number which is 6 022 x 10 23 atoms or molecules Stoichiometric coefficients The coefficients in a balanced chemical equation Stoichiometry An accounting of atoms in a chemical reaction REVIEW OF COUNTING ATOMS Subscripts number of atoms in a formula Coefficients TOTAL number of molecules or compounds Ex Ca3 PO4 2 Ex 3Ca3 PO4 2 PRACTICE How many oxygen atoms in each NH4NO3 Al2 SO4 3 3Al2 SO4 3 The MOLE mol
More picture related to Stoichiometry Packet Answer Key

Answer Key To Stoichiometry Problem Set
https://s3.studylib.net/store/data/007073935_1-92a28c3b7be7e7d33f3739b024710f5a-768x994.png
Pogil Stoichiometry Worksheet Answer Key
https://i2.wp.com/lh5.googleusercontent.com/proxy/Db_89uXW3YzgbHD02uF92kJLDWTOpipX5l7e86NXZ7eUe8deYAIUtyxL0li8uDwoJXnQ6IYJZrk7-z00IdbPKRxkRz_UCEkEIenf8iOqArTPdT93SXUgj2zKGQ_2XrcX7hXolQI1QnwEXzHp8W1u7mPW9oDmHU7SF6cZXm0=s0-d
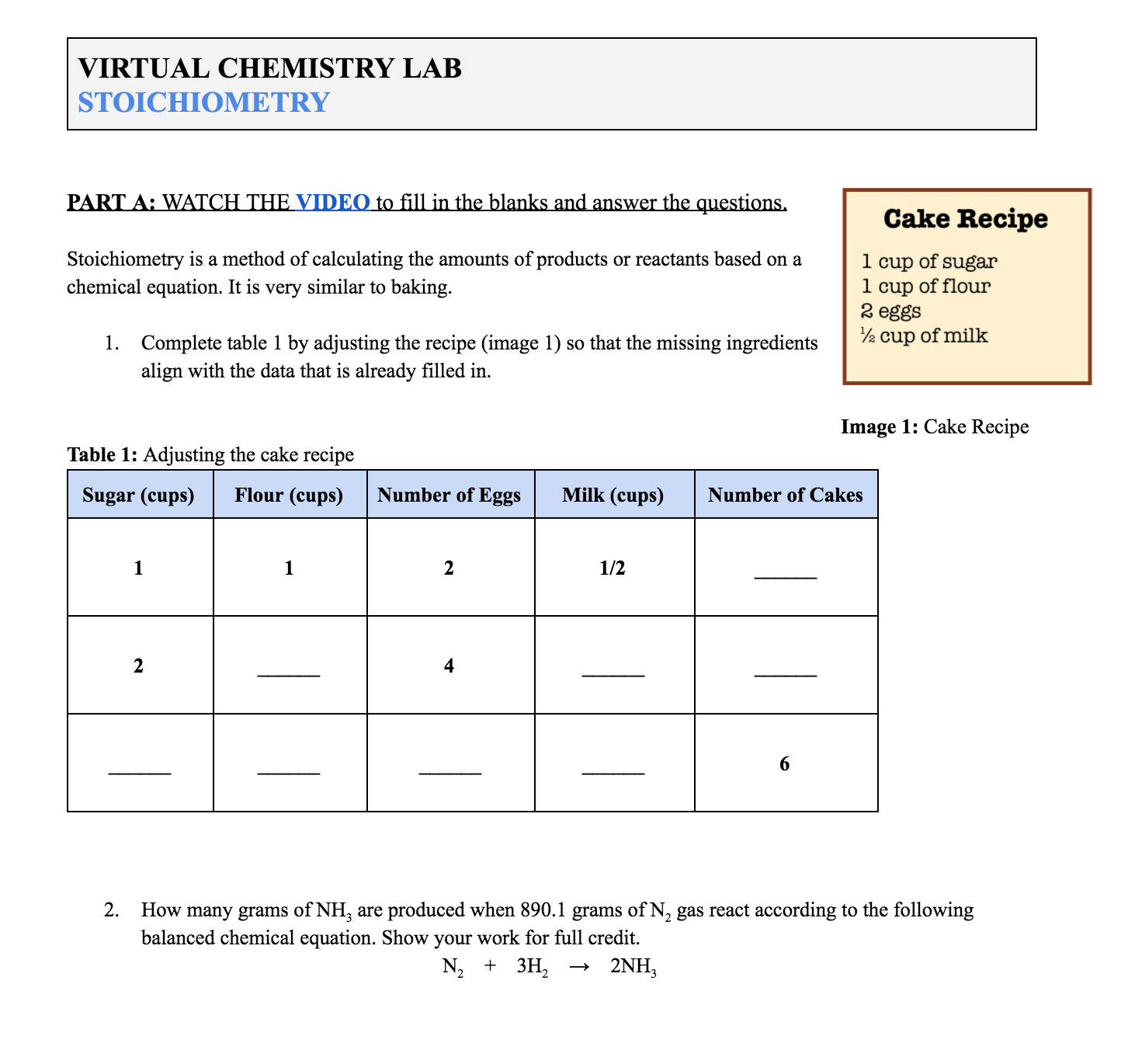
Basic Stoichiometry Phet Lab Answers 2 This Basic Stoichiometry
https://www.chemedx.org/sites/www.chemedx.org/files/stoichlab2.png
Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N 2 3 F 2 2 NF 3 2 2 C 6 H 10 17 O 2 12 CO 2 10 H 2 O 3 1 HBr 1 KHCO 3 1 H 2 O 1 KBr 1 CO 2 4 2 GaBr 3 3 Na 2 SO 3 and the reagent that leads to this answer is the limiting reagent Both
Multiplying the number of moles of H A 2 SO A 4 by this factor gives us the number of moles of NaOH needed 3 16 10 2 mol H 2 SO 4 2 mol NaOH 1 mol H 2 SO 4 6 32 10 2 mol NaOH Notice how we wrote the mole ratio so that the moles of H A 2 SO A 4 cancel out resulting in moles of NaOH as the final units PRACTICE PACKET Unit 7 Moles Stoichiometry 5 chempride weebly ADDITIONAL PRACTICE LESSON 1 Find the gram formula mass of the following Show all work 1 MgO 5 Ca OH 2 2 NaHCO 3 6 CH 4 3 C 6 H 12 O 6 7 NH 3 4 Al 2 O 3 8 H 2 O 2 ASSESS YOURSELF ON THIS LESSON 10

Stoichiometry Worksheet Answer Key Db excel
https://db-excel.com/wp-content/uploads/2019/09/stoichiometry-worksheet-answer-key-2.jpg
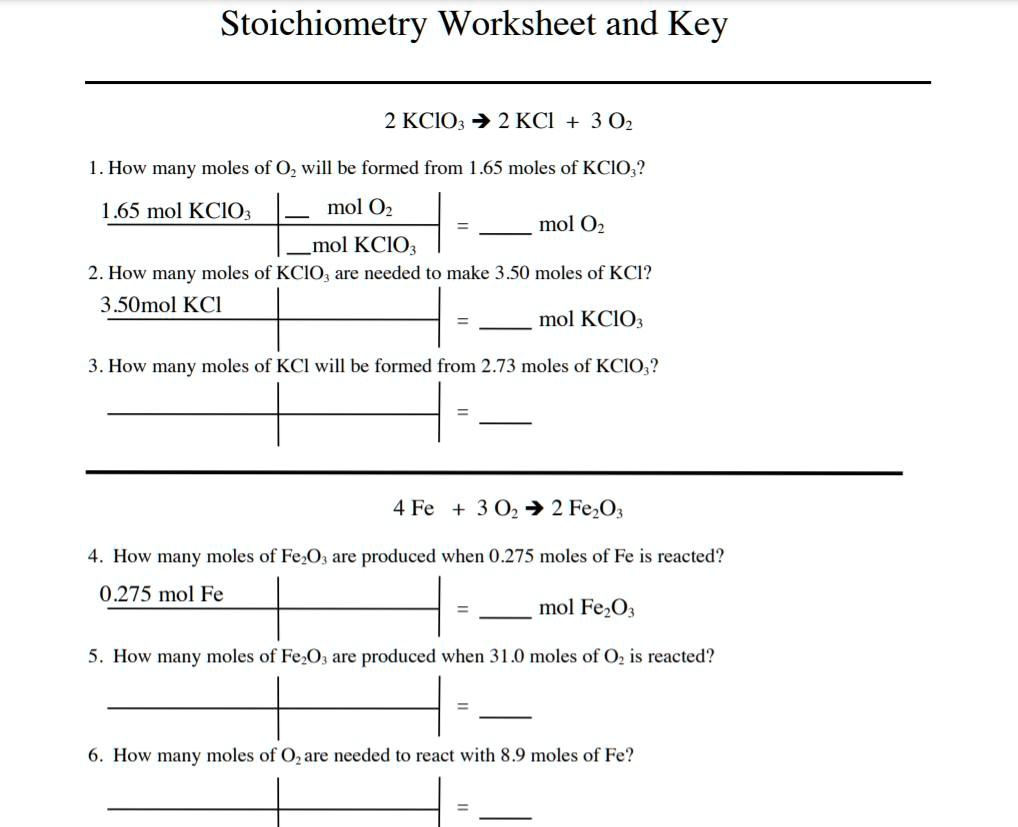
Stoichiometry Worksheet Answer Key
https://cdn.numerade.com/ask_images/1ac6ca7de1ee4f6b8029cb65414a8499.jpg
Stoichiometry Packet Answer Key - Stoichiometry An accounting of atoms in a chemical reaction REVIEW OF COUNTING ATOMS Subscripts number of atoms in a formula Coefficients TOTAL number of molecules or compounds Ex Ca3 PO4 2 Ex 3Ca3 PO4 2 PRACTICE How many oxygen atoms in each NH4NO3 Al2 SO4 3 3Al2 SO4 3 The MOLE mol