Stoichiometry Mole To Mass Problems Worksheet Answers A How many moles of dinitrogen tetrahydride are required to produce 57 moles of nitrogen B How many moles of dinitrogen tetroxide are required to produce 57 moles of nitrogen C How many moles of water are produced when 57 moles of nitrogen are made 3 Calculate the mass of aluminum oxide produced when 3 75 moles of aluminum burn in oxygen
Converting moles and mass The molecular weight of sodium chloride NaCl is 58 44 g mol How many moles of salt are in 13 8 g of sodium chloride Express the answer using 3 significant figures Stuck Use a hint Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Example 1 How many grams of hydrogen gas are needed to react completely with 54 0 g of oxygen gas given the following unbalanced chemical reaction 1 Balance the chemical equation 2 Convert grams of the substance given 54 0 g 32 0 g mol 1 6875 mol of O Note the use of 32 0 and not 16 0
Stoichiometry Mole To Mass Problems Worksheet Answers

Stoichiometry Mole To Mass Problems Worksheet Answers
http://www.worksheeto.com/postpic/2015/10/mole-stoichiometry-worksheet-answers_224038.png
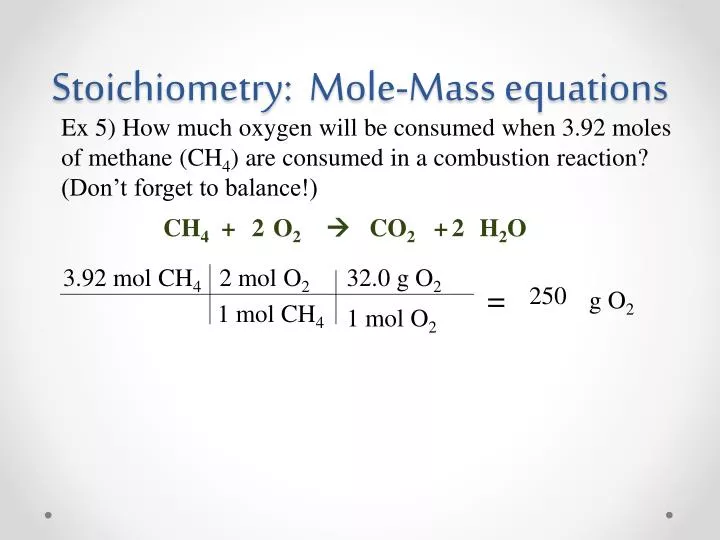
PPT Stoichiometry Mole Mass Equations PowerPoint Presentation Free
https://image2.slideserve.com/4011225/stoichiometry-mole-mass-equations-n.jpg
Stoichiometry Part 2 Worksheet Free Download Gmbar co
https://media.cheggcdn.com/media/f6b/f6b990df-cc62-4524-988f-341d7f51f886/php3mB8qv
Step 1 Convert known reactant mass to moles In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio we first need to know the quantity of H A 2 SO A 4 in moles We can convert the 3 10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 98 08 g mol You do this by multiplying the moles by the molar mass of the substance This technique is covered in the mole section of the ChemTeam Click this link to go to the proper mole file for review Here is the equation we ll use for the first three examples 2KClO 2KCl 3O Example 1 1 50 mol of KClO decomposes
Google Classroom You might need Calculator Periodic table Table salt or sodium chloride can be decomposed into sodium metal and chlorine gas by running electricity through molten NaCl A Downs cell produces sodium and chlorine from molten sodium chloride The balanced equation for this reaction is 2 NaCl l 2 Na l Cl A 2 g A If you have 5 50 mol of CaC2 how much C2H2 do you get 143g C2H2 b How many moles of water are needed when 65 0g of CaC2 have reacted 2 03 mol H2O 4 In photosynthesis water reacts with carbon dioxide to give oxygen and glucose C6H12O6 Write and balance the chemical equation
More picture related to Stoichiometry Mole To Mass Problems Worksheet Answers

Mole Mass Problems Worksheet Answers Ft Grade Math Db excel
https://db-excel.com/wp-content/uploads/2019/09/mole-mass-problems-worksheet-answers-ft-grade-math.jpg

Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers Laabeja
https://i.redd.it/55y9udo3q5r21.jpg
The Mole Worksheet Chemistry Answers
http://www.worksheeto.com/postpic/2013/12/mass-to-mole-stoichiometry-worksheet-answer-key_208083.jpg
This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula Problem Set One Problem Set Two Mole Mole Problems Worksheet Mole Mass Problems Worksheet Mole Mole and Mole Mass Problems Mixed Problems Mole Mole and Mole Mass Worksheet Challenge Problem Stoichiometry This semester begins with the introduction of the mole
Problem Set ST4 Mole to Mole to Mass Stoichiometry 2 To perform two step conversions to determine the mass of a reactant or product from knowledge of the number of moles of a reactant or product involved in the reaction or vice versa Includes 6 problems Problem Set ST5 Introduction to Mass to Mass Stoichiometry First let us work the problem in stepwise fashion We begin by converting the mass of CH 4 to moles of CH 4 using the molar mass of CH 4 16 05 g mol as the conversion factor 100 0 g CH 4 1 mol CH 4 16 05 g CH 4 6 231 mol CH 4 Note that we inverted the molar mass so that the gram units cancel giving us an answer in moles

Stoichiometry Worksheet 1 Mass Mass
https://images.sampletemplates.com/wp-content/uploads/2016/11/17162322/Mass-to-Mass-Stoichiometry-Worksheet.jpg

Mole And Mass Stoichiometry Problems
https://s3.studylib.net/store/data/007734847_2-ccaf4ade60b8a5a6d3eb03b9a1dd62e3-768x994.png
Stoichiometry Mole To Mass Problems Worksheet Answers - Stoichiometry Worksheet answers Name Date Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C4H10 13 O2 8 CO2 10 H2O show what the following molar ratios should be a C4H10 O2 b O2 CO2 c O2 H2O d C4H10 CO2 e C4H10 H2O 2
