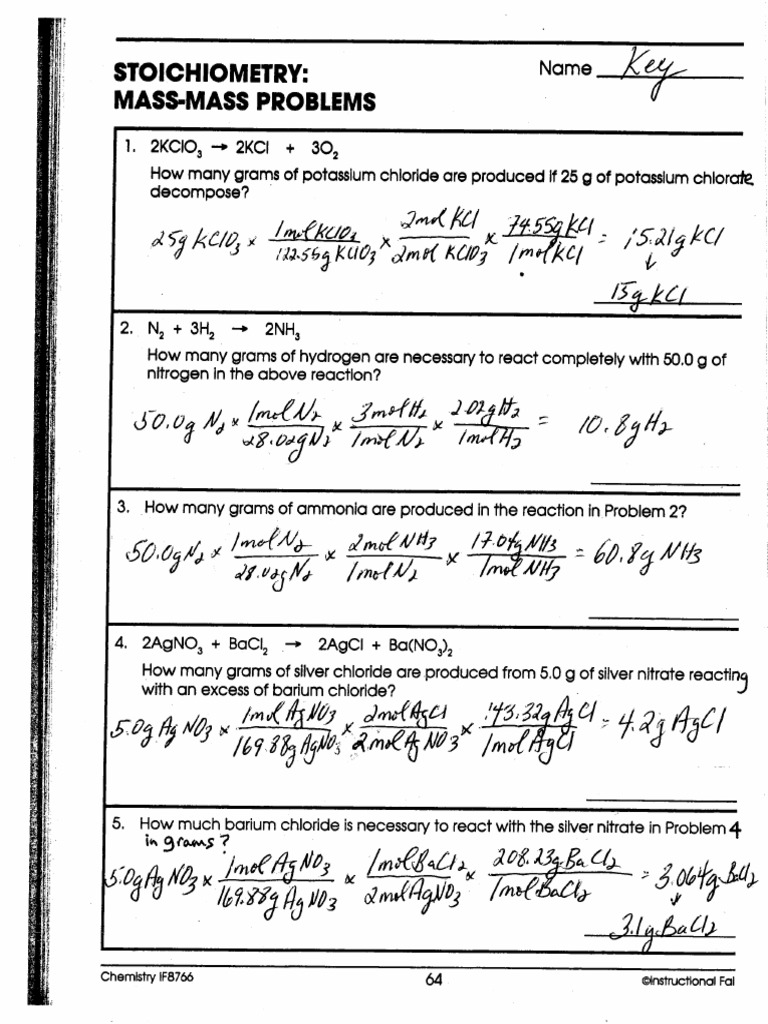
Stoichiometry Mass To Mass Worksheet Answers How many moles of water are produced when 57 moles of nitrogen are made 3 Calculate the mass of aluminum oxide produced when 3 75 moles of aluminum burn in oxygen Answers 1A 30 mol Ag 1B 30 mol AgNO3 1C 20 mol H2O 1D 10 mol NO 2A 38 mol N2H4 2B 19 mol N2O4 2C 76 mol H2O
STOICHIOMETRY WORKSHEET 1 MASS MASS 1 Determine the mass of lithium hydroxide produced when 0 38 grams of lithium nitride reacts with water according to the following unbalanced chemical equation Li 3N s H 2O l NH 3 g LiOH aq 2 What mass of sodium chloride is produced when chlorine gas reacts with 0 29 grams of sodium iodide Example 1 How many grams of hydrogen gas are needed to react completely with 54 0 g of oxygen gas given the following unbalanced chemical reaction 1 Balance the chemical equation 2 Convert grams of the substance given 54 0 g 32 0 g mol 1 6875 mol of O Note the use of 32 0 and not 16 0
Stoichiometry Mass To Mass Worksheet Answers
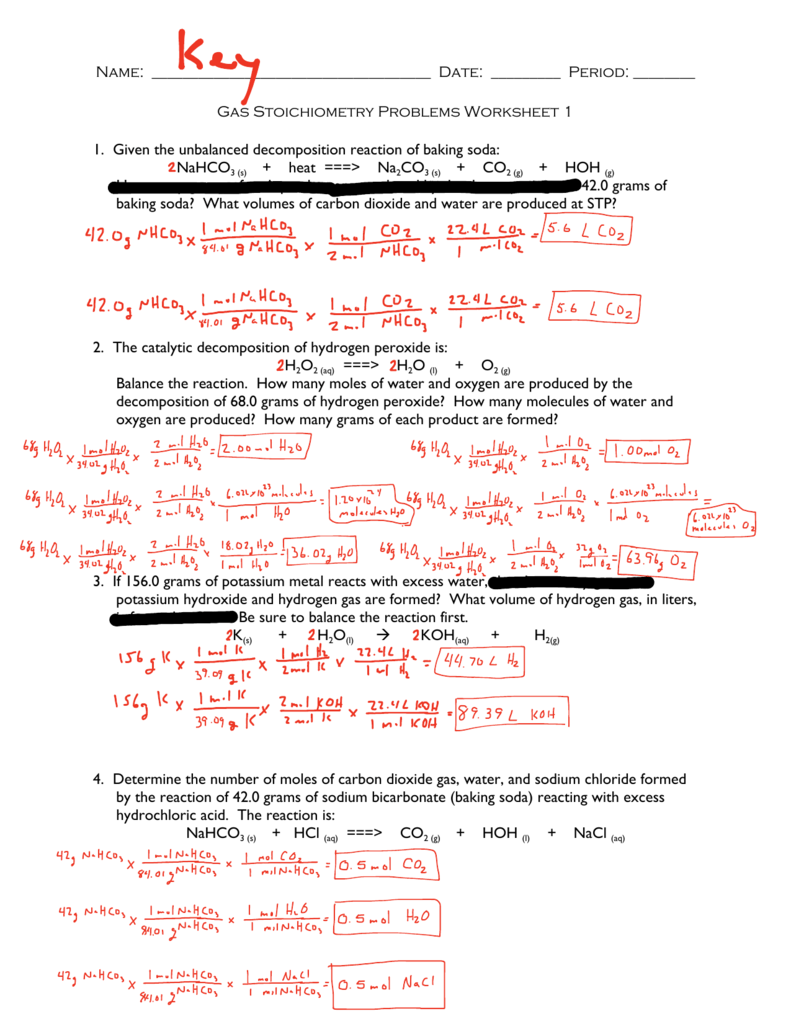
Stoichiometry Mass To Mass Worksheet Answers
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png

Stoichiometry Worksheet Answer Key
https://s3.studylib.net/store/data/008365019_1-81aefc395fc4a1be28e4f289b3863708.png

Stoichiometry Mass To Mass Worksheet Answers
https://i.ytimg.com/vi/anh4rGF_3QU/maxresdefault.jpg
Mass to Mass or Mass to Mole Conversions Objective Given the mass one species be able to predict the mass another species consumed or produced from a balanced chemical equation Technique This is a three step process which should be done in one equation which uses three conversion factors Conversion Factor 1 Use molar mass to convert mass Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C 4H 10 13 O 2 8 CO 2 10 H 2O show what the following molar ratios should be a C 4H 10 O 2 b O 2 CO 2 c O 2 H 2O d C 4H What mass of iron is needed to react with 16 0 grams of sulfur 27 87 g Fe b How many grams of FeS are produced
The reaction is Fe O 3C 2Fe 3CO Solution 1 Determine moles of Fe 2 O 3 used 25000 g 159 694 g mol 156 5494 mol Fe O 2 Use a ratio and proportion to determine moles of Fe produced and the g Fe They cancel each other because the UNIT is grams on both of them Chemistry Stoichiometry Problem Sheet 2 Directions Solve each of the following problems Show your work including proper units to earn full credit CaCl2 AgNO3 Ca NO3 2 AgCl How many grams of silver chloride are produced when 45 g of calcium chloride react with excess silver nitrate g AgCl 45 g CaCl 2
More picture related to Stoichiometry Mass To Mass Worksheet Answers
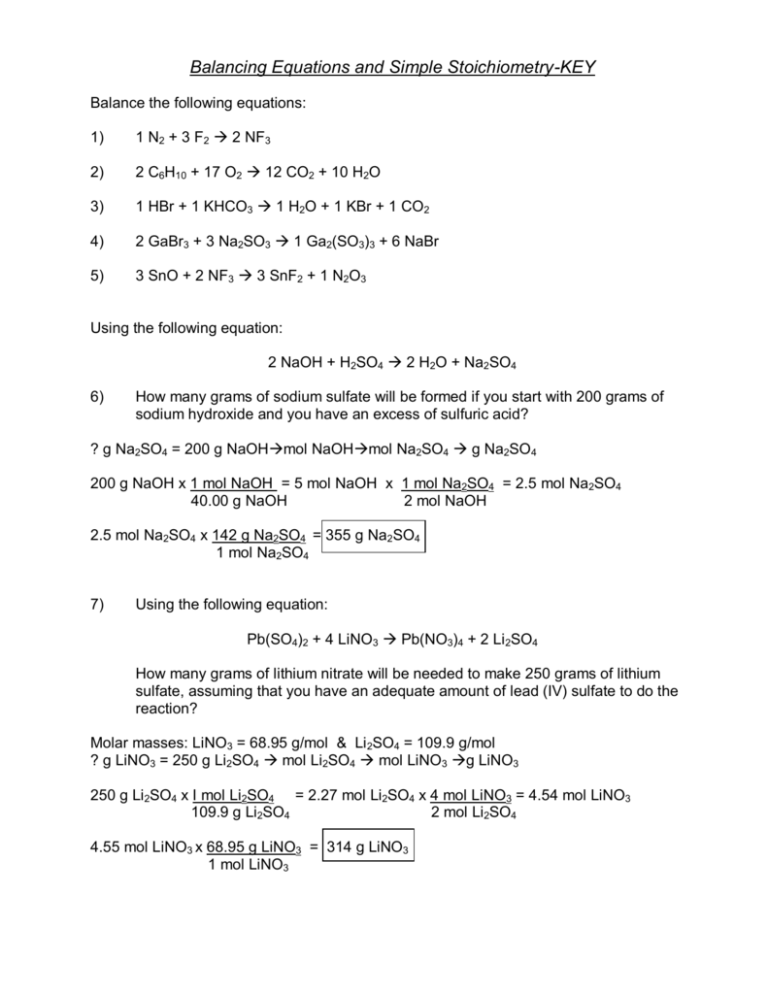
KEY Solutions For The Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008711874_1-d12f29b6b62688faeae95fc3bd7c9543-768x994.png

Stoichiometry Problems Worksheet Answers Educational Worksheet
https://s3.studylib.net/store/data/008548006_1-87998e5b1b015fdc34a74176a67dd4ab.png
Mass Mass Stoichiometry Worksheet Detailed Answer Key Distance Learning
https://cdnapisec.kaltura.com/p/2172211/thumbnail/entry_id/1_mxaiavwc/def_height/500/def_width/500/
To do this start by dividing the smallest number of moles into each of the numbers of moles of elements i e set the smallest number to 1 This may yield integers or it may yield decimal results that correspond closely to rational fractions For example 1 25 2 75 1 2 5 11 NAME Question Answer Methane burns readily in oxygen to produce carbon dioxide and water according to the equation CH4 g 2O2 g CO2 g 2H2O l How many mol of CO2 will be produced by the complete oxidation of 4 5 mol of CH4 in excess oxygen 2 When chlorine gas is bubbled through water a solution of hydrochloric acid is produced
CHM 130 Stoichiometry Worksheet The following flow chart may help you work stoichiometry problems Remember to pay careful 2 CO 2 g A Calculate the mass of ethanol produced if 500 0 grams of glucose reacts completely B Calculate the volume of carbon dioxide gas produced at STP if 100 0 grams of glucose reacts C If 17 5 moles of Sample Problem Mass Mass Stoichiometry Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation NH 4 NO 3 s N 2 O g 2 H 2 O l In a certain experiment 45 7 g of ammonium nitrate is decomposed Find the mass of each of the products formed Step 1 List the known quantities and plan the
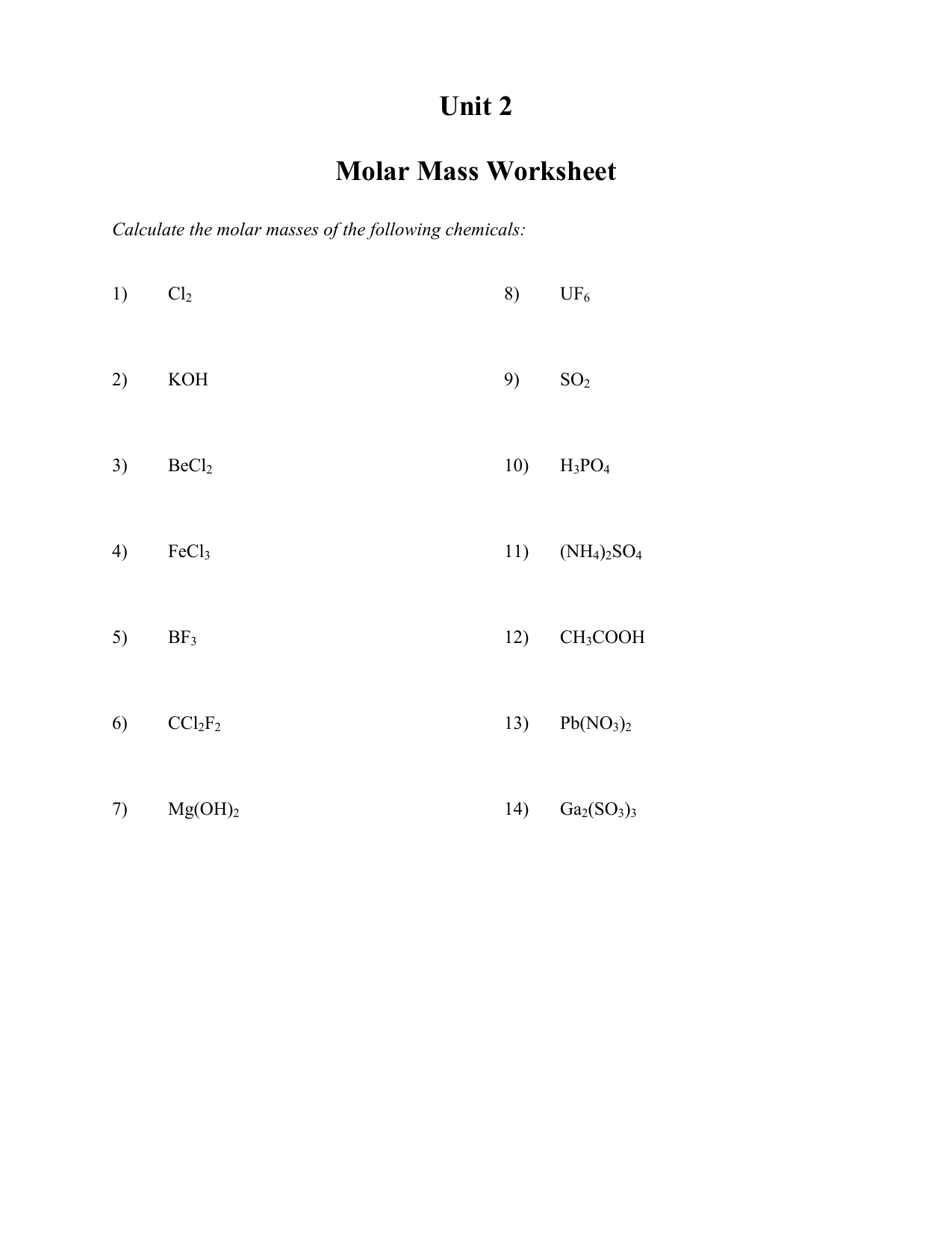
Worksheets For Unit 2 Molar Mass To Stoichiometry
https://s3.studylib.net/store/data/025213414_1-b6c39322375ae31656aeb2aa3dd5357c.png
Stoich Mass Mass Problems Answer Key PDF
https://imgv2-1-f.scribdassets.com/img/document/483470203/original/e7f36787bd/1685088834?v=1
Stoichiometry Mass To Mass Worksheet Answers - Stoichiometry Worksheet 2 mole mass mass mole problems 1 N 2 2O 2 N 2 O 4 a If 15 0g of N 2 O 4 What is the mass of potassium nitrate that is produced when 2 04 moles of potassium phosphate react 2 04 mol K 3 PO 4 Balance and answer the following questions a How many grams of NaCl are produced when 20 00mol of NaClO 3