Molecular Formula Worksheet Answers This 10 question practice test deals with finding the molecular formula of chemical compounds A periodic table will be required to complete this test Answers appear after the final question Question 1 An unknown compound is found to contain 40 0 carbon 6 7 hydrogen and 53 3 oxygen with a molecular mass of 60 0 g mol
Empirical and Molecular Formula Worksheet ANSWER KEY Write the empirical formula for the following compounds 1 C6H6 6 C8H18 7 WO 2 8 C2H6O2 9 X39Y13 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula
Molecular Formula Worksheet Answers

Molecular Formula Worksheet Answers
https://www.chemistryworksheet.com/wp-content/uploads/2022/10/empirical-and-molecular-formula-worksheets-answers.jpg

11 Best Images Of Molecular Formula Worksheet With Answers Molecular
http://www.worksheeto.com/postpic/2014/05/molecular-and-empirical-formula-worksheet_427081.png
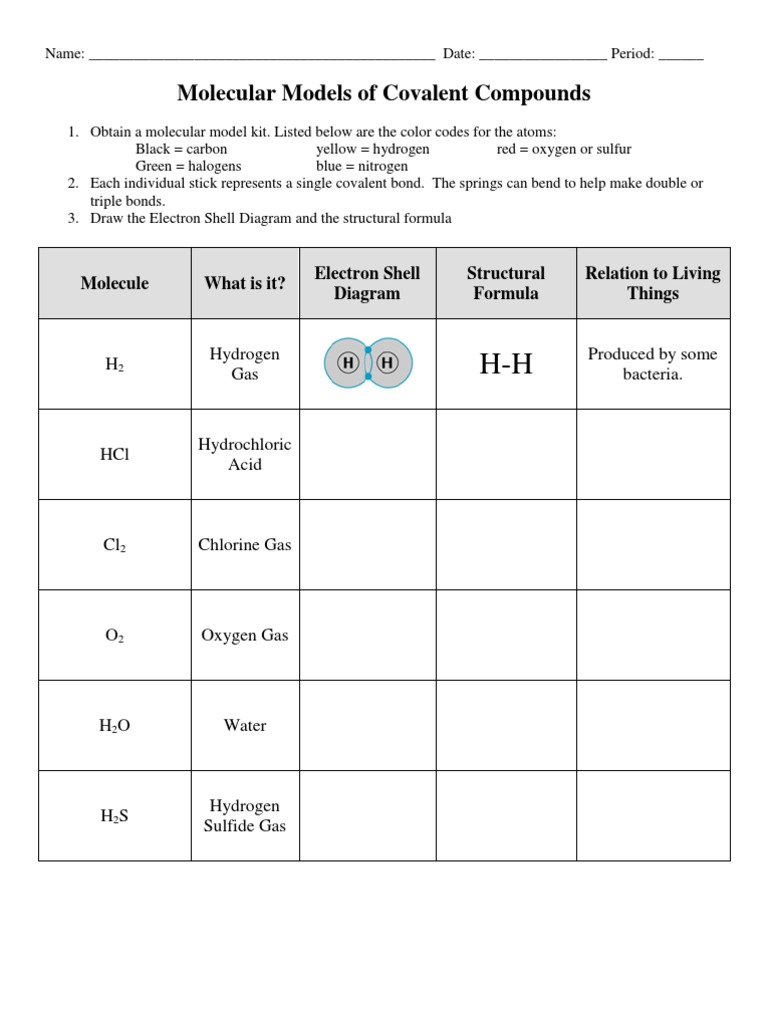
Molecular Models Of Covalent Compounds Worksheet
https://imgv2-2-f.scribdassets.com/img/document/124934588/original/f116bba140/1618892100?v=1
Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula Empirical and Molecular Formulas Worksheet Objectives be able to calculate empirical and molecular formulas Empirical Formula 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O
PROBLEM 4 4 1 10 4 4 1 10 Determine the number of moles of compound and the number of moles of each type of atom in each of the following a 25 0 g of propylene C 3 H 6 b 3 06 10 3 g of the amino acid glycine C 2 H 5 NO 2 c 25 lb of the herbicide Treflan C 13 H 16 N 2 O 4 F 1 lb 454 g Determine the molecular formula for each compound whose percentage composition is shown below 6 84 9 Hg and the remainder Cl with a molecular weight of 472 2 g mol Answers to Worksheet 8 Empirical Formulas To calculate empirical formulas follow the steps outlined below assume percentages given in
More picture related to Molecular Formula Worksheet Answers
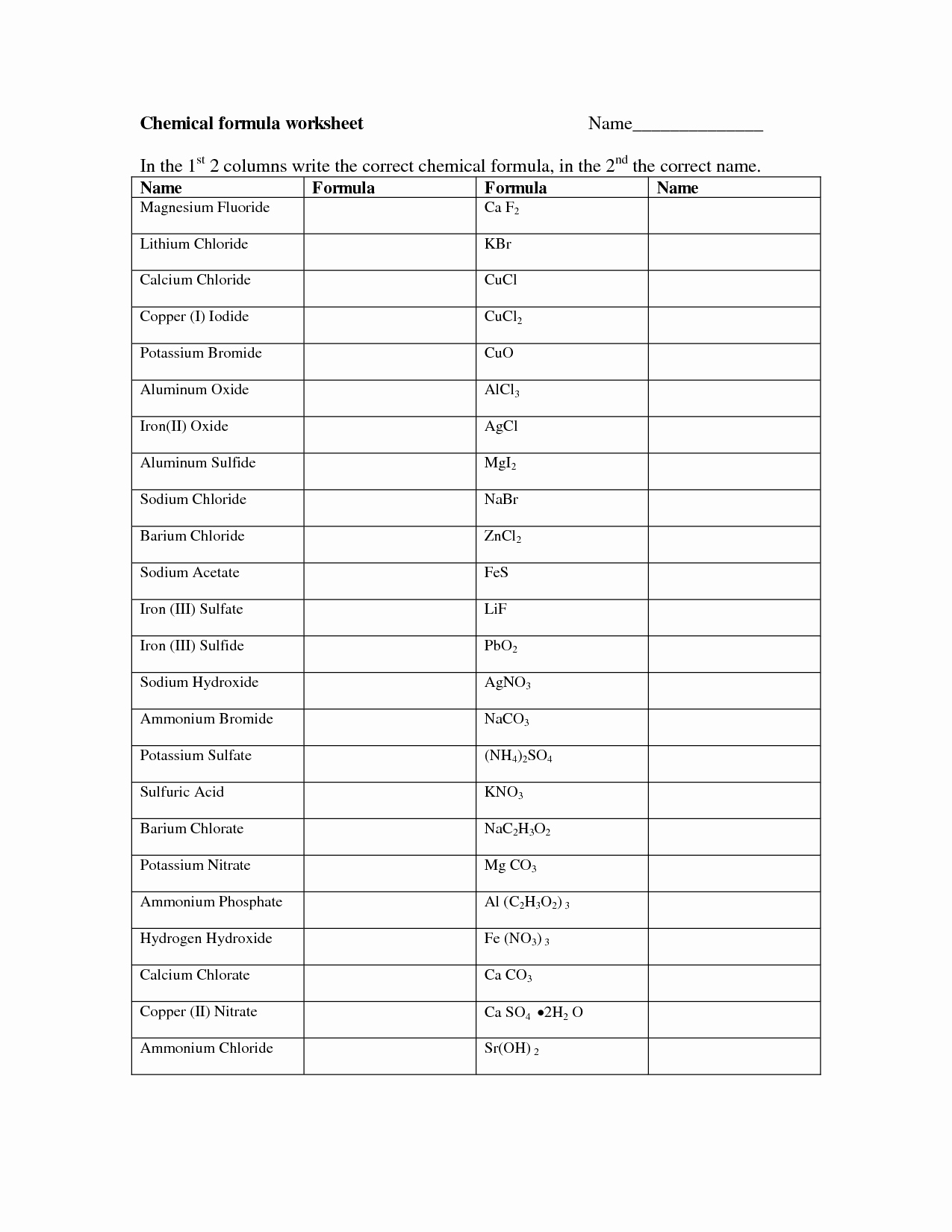
50 Chemical Formula Worksheet Answers
https://chessmuseum.org/wp-content/uploads/2019/10/chemical-formula-worksheet-answers-awesome-14-best-of-easy-write-ionic-formulas-worksheet-of-chemical-formula-worksheet-answers.png

Empirical And Molecular Formula Worksheet Answers Free Printable
https://www.problemsworksheets.com/wp-content/uploads/2023/03/empirical-formula-worksheet-answers-free-download-qstion-co.jpg
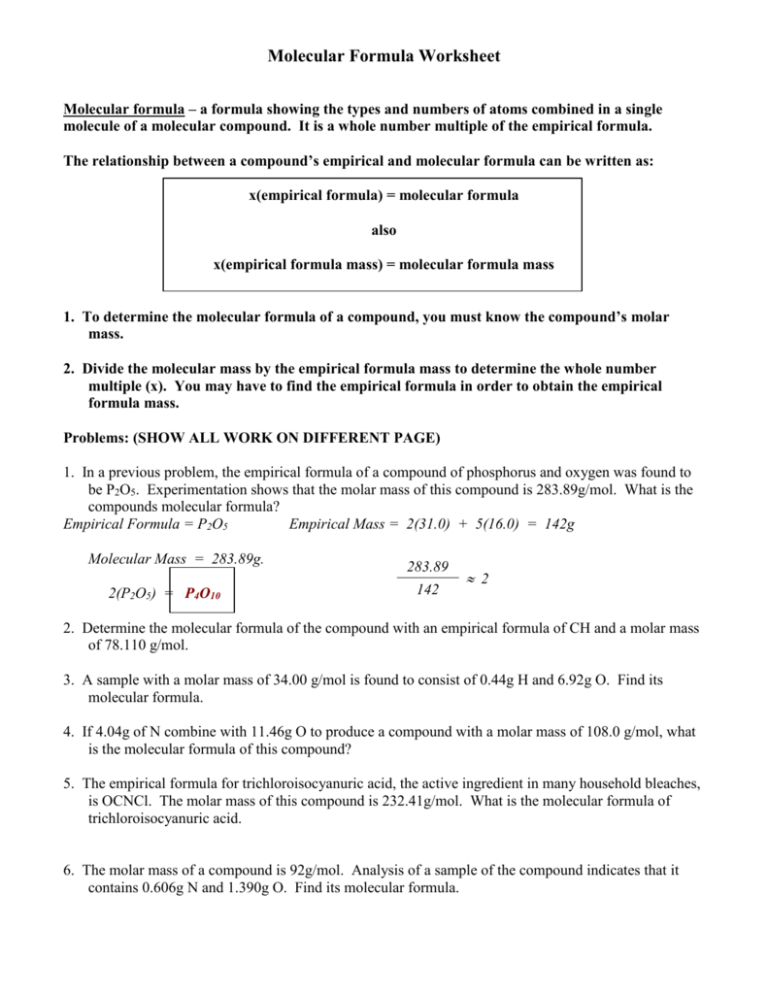
Molecular Formula Worksheet
https://s3.studylib.net/store/data/008992102_1-022f53585d7a7efb11e3a9f060aba7b9-768x994.png
MOLECULAR FORMULAS To determine the molecular formula for a compound 1 The molecular weight is always a multiple of the empirical formula weight i e M W n E F W To determine n divide the given molecular weight by the empirical formula weight This work is licensed under a Creative Commons Attribution 4 0 International License a c
Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER Write the empirical formula for the following compounds 1 C 6H 6 2 C8H18 3 WO2 4 C2H6O2 5 X 39Y 13 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound Question Empirical Molecular Formula Practice Worksheet Directions Find the empirical AND molecular formulas given the percent compositions or masses SHOW YOUR WORK to receive full credit 1 26 4 Carbon 3 3 Hydrogen 70 3 Oxygen Molar Mass 91 0 g mol Empirical Formula Molecular Fornmila 2 81 8 grams Carbon 18 2 grams Hydrogen Molar

12 Naming Molecular Compounds Worksheet Answers Worksheeto
https://www.worksheeto.com/postpic/2009/04/binary-molecular-compounds-worksheet-answers_554202.png

Molecular Shape And Polarity Worksheet Answers
https://i.pinimg.com/originals/4b/3a/a5/4b3aa542270b832b0b8c8127282fb287.jpg
Molecular Formula Worksheet Answers - Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula