Polyatomic Ions Packet Sulfite SO 3 2 dichromate Cr 2 O 7 2 triiodide I 3 The naming of ionic compounds that contain polyatomic ions follows the same rules as the naming for other ionic compounds simply combine the name of the cation and the name of the anion Do not use numerical prefixes in the name if there is more than one polyatomic ion the
Oxyanions You may have noticed that most of the polyatomic ions in Table 5 7 1 5 7 1 carry a negative charge and contain the element oxygen Anions that contain the element oxygen are called oxyanions Many oxyanions belong to a series where the number of oxygen atoms in the ion varies as shown in Table 5 7 2 5 7 2 Polyatomic ions are charged groups of atoms An example is ammonium ion NH 4 It has five atoms one nitrogen and four hydrogens that share a charge of 1 The polyatomic ions remain intact and parentheses may be required when using subscripts
Polyatomic Ions Packet
Polyatomic Ions Packet
https://educationdetailsonline.com/wp-content/uploads/2019/05/Polyatomic-Ions-2.jpg

Most Common Polyatomic Ions List Cheat Sheet StudyPK
https://www.studypk.com/wp-content/uploads/2020/05/Most-Common-Polyatomic-Ions-List-Cheat-Sheet-1583x2048.png

Polyatomic Ions By Cam Diagram Quizlet
https://o.quizlet.com/hffFltbgrA0UBRCVa27qeQ_b.jpg
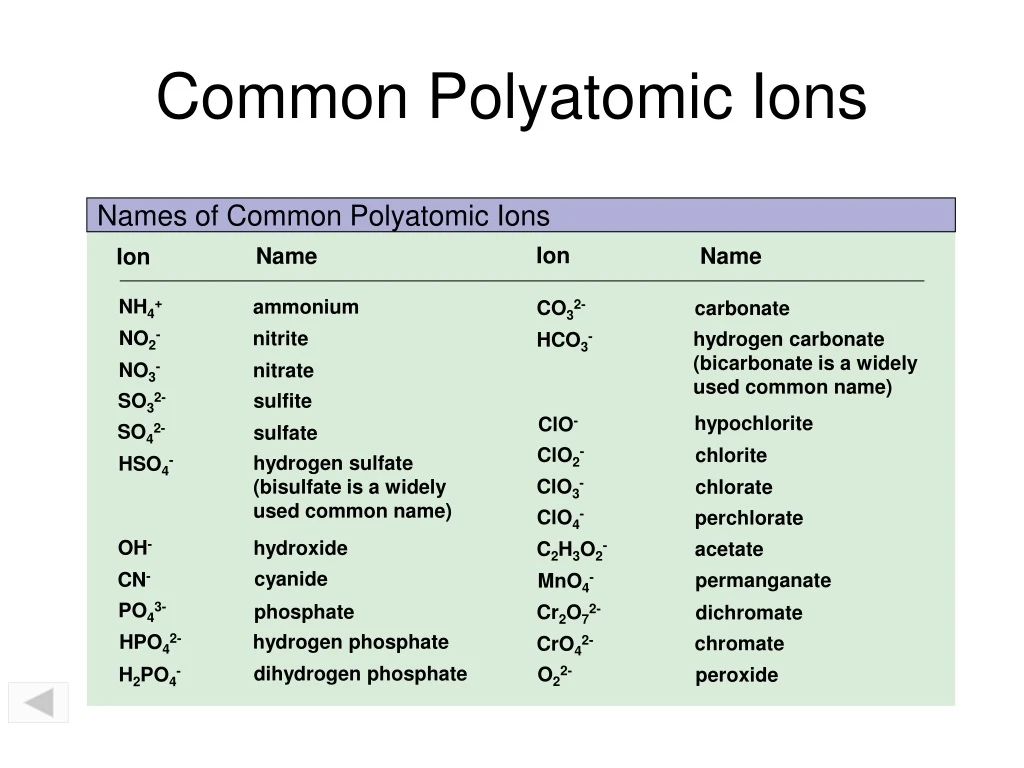
Common Polyatomic Ions 4 Charge P2O7 4 pyrophosphate Most commonly encountered ions in bold Polyatomic Ions A group of atoms held together by covalent bonds found in ionic compounds Know memorize recognize names formulas and charges General Information Recognizing Ionic vs Covalent Compounds Compounds Compounds Polyatomic ions Intro to polyatomic ions Google Classroom Learn what polyatomic ions are and how they bond Some ions consist of a single atom with a net charge They re called monatomic ions Examples include Na O 2 and Cl Other ions consist of a molecule a group of atoms covalently bonded together with a net charge
A polyatomic ion also known as a molecular ion is a covalent bonded set of two or more atoms or of a metal complex that can be considered to behave as a single unit and that has a net charge that is not zero 1 The term molecule may or may not be used to refer to a polyatomic ion depending on the definition used Rules for Naming Ionic Compounds Containing Polyatomic Ions Polyatomic ions are ions which consist of more than one atom For example nitrate ion NO 3 contains one nitrogen atom and three oxygen atoms The atoms in a polyatomic ion are usually covalently bonded to one another and therefore stay together as a single charged unit
More picture related to Polyatomic Ions Packet
PPT Common Polyatomic Ions PowerPoint Presentation Free Download
https://image4.slideserve.com/9103373/common-polyatomic-ions-n.jpg

Polyatomic Ions Worksheet Sample Free Download CompoundWorksheets
https://www.compoundworksheets.com/wp-content/uploads/2023/03/polyatomic-ions-worksheet-sample-free-download-19.png

Polyatomic Ion Charts Find Word Templates
https://i1.wp.com/www.findwordtemplates.com/wp-content/uploads/2016/08/Polyatomic-Ion-Chart-5.jpg?w=960&ssl=1
Poly atomic ions are covalent compounds that have an overall charge and therefore are held together through the electrostatic attraction of ionic bonding to positively charged ions called cations With the exception of ammonium N H 4 these ions carry a negative Examples include sulfate SO 2 4 nitrate N O 3 and phosphate P O 3 4 Polyatomic ions are ions that contain more than one element This polyatomic ions list contains many common ions grouped by charge Each entry contains the ion s name molecular formula and chemical structure 1 Polyatomic Ions
If a polyatomic ion is involved remember that more than one polyatomic is shown in parentheses i e DO NOT multiply the charge of the polyatomic ion with the subscript of the atoms in a polyatomic ion There is only ONE Cu and ONE S04 so get the charge for the Cu based on the S04 The new sum is 2 2 1 0 therefore the formula is CaBr2 A simpler way to understand how to write formulas is to use the crisscross reduce method If there is only one atom of an element in the compound you do not need to put the number 1 behind it The following are examples

Standard Polyatomic Ions Chart Free Download
https://www.formsbirds.com/formimg/polyatomic-ions-chart/6824/standard-polyatomic-ions-chart-l1.png

How To Memorize Polyatomic Ions Chemical Formulas SuperHuman Academy
https://superhumanacademy.com/wp-content/uploads/2018/08/Naming-polyatomic-ions.png
Polyatomic Ions Packet - Common Polyatomic Ions 4 Charge P2O7 4 pyrophosphate Most commonly encountered ions in bold Polyatomic Ions A group of atoms held together by covalent bonds found in ionic compounds Know memorize recognize names formulas and charges General Information Recognizing Ionic vs Covalent Compounds Compounds Compounds