Molecular Formula Worksheet Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula
This 10 question practice test deals with finding the molecular formula of chemical compounds A periodic table will be required to complete this test Answers appear after the final question Question 1 An unknown compound is found to contain 40 0 carbon 6 7 hydrogen and 53 3 oxygen with a molecular mass of 60 0 g mol Determine the empirical and molecular formula for chrysotile asbestos Chrysotile has the following percent composition 28 03 Mg 21 60 Si 1 16 H and 49 21 O The molar mass for chrysotile is 520 8 g mol Answer Mg 3 Si 2 H 3 O 8 empirical formula Mg 6 Si 4 H 6 O 16 molecular formula
Molecular Formula Worksheet

Molecular Formula Worksheet
https://teachsimplecom.s3.us-east-2.amazonaws.com/images/empirical-and-molecular-formula-practice/image-1653688677256-2.jpg

Empirical And Molecular Formula Practice Problems
https://s3.studylib.net/store/data/007884987_2-0d8a2dde770adff75de1113cf0fd4dd0.png
Empirical And Molecular Formula Worksheet 6 10 Worksheets For
https://s3.studylib.net/store/data/025236217_1-3445983110510a5896d4bfb99ff9c6b6-768x994.png
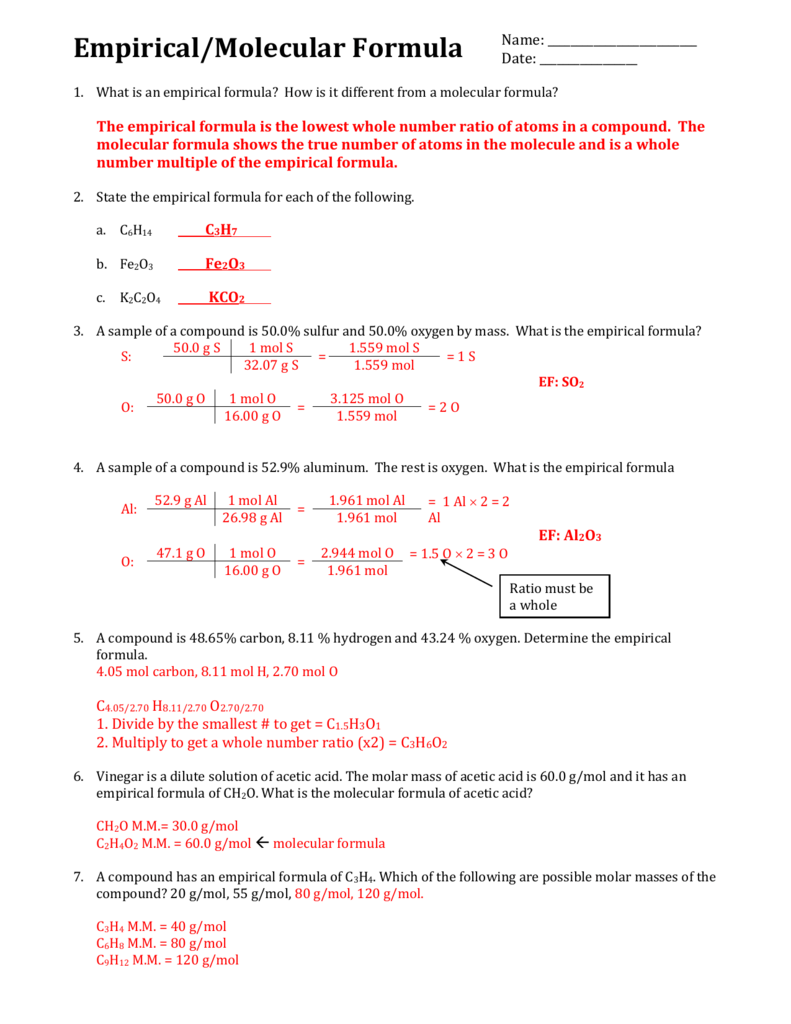
An empirical formula refers to the simplest ratio of elements that is obtained from a chemical formula while a molecular formula is calculated to show the actual formula of a molecular compound 2 Construct a flowchart showing how you would determine the empirical formula of a compound from its percent composition Objectives be able to calculate empirical and molecular formulas Empirical Formula 1 What is the empirical formula of a compound that contains 0 783g of Carbon 0 196g of Hydrogen and 0 521g of Oxygen 2 What is empirical formula of a compound which consists of 89 14 Au and 10 80 of O
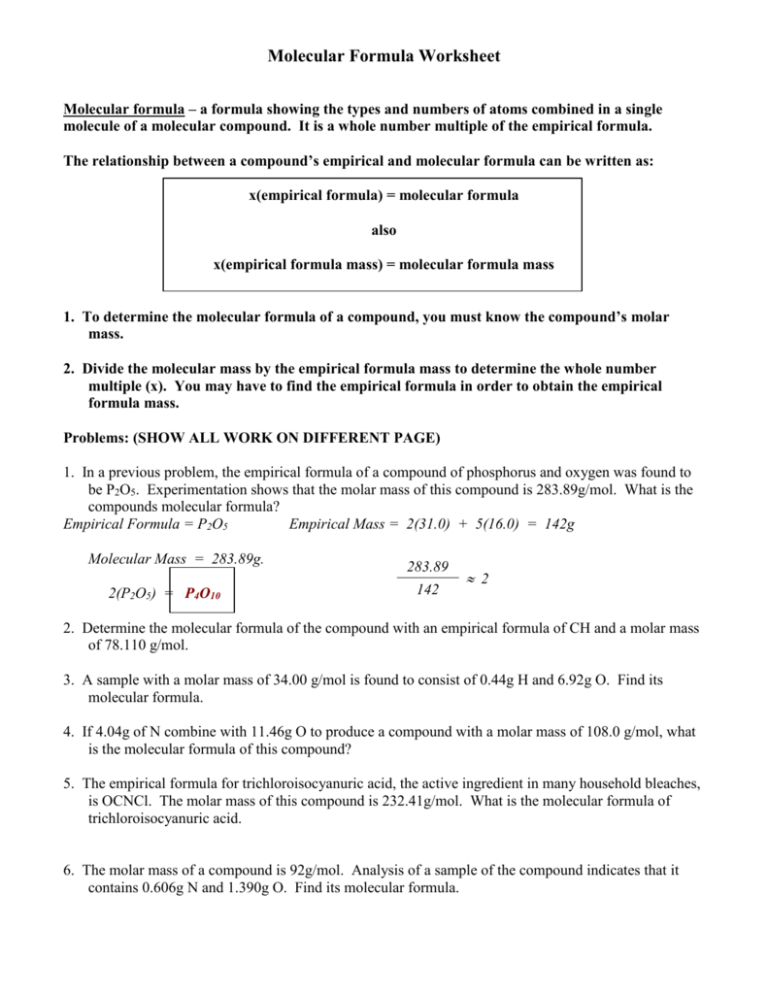
MOLECULAR FORMULAS To determine the molecular formula for a compound 1 The molecular weight is always a multiple of the empirical formula weight i e M W n E F W To determine n divide the given molecular weight by the empirical formula weight This work is licensed under a Creative Commons Attribution 4 0 International License Empirical and Molecular Formulas Exercise 7 2 3 1 7 2 3 1 For each formula below give the empirical formula Sometimes the formula given is the same as the empirical sometimes it is different a C 3 H 6 O 3 b N 2 O 4 c Mg 3 N 2 d C 7 H 14 O 2 e P 2 O 5
More picture related to Molecular Formula Worksheet
30 Empirical And Molecular Formulas Worksheet Support Worksheet
https://s3.studylib.net/store/data/006993380_1-5ca613f11ecd39eca6c06abf3fe85484.png

Free Printable Empirical And Molecular Formula Worksheets
https://www.chemistrylearner.com/wp-content/uploads/2023/11/Empirical-and-Molecular-Formula-Worksheet-Chemistry-724x1024.webp

Free Empirical molecular Formula Practice Worksheets
https://storage.googleapis.com/worksheetzone/image/63982c9316529235c9236b85/empirical-formulas-molecular-formulas-w1000-h1294-preview-3.png
Empirical and Molecular Formula Worksheet SHOW WORK ON A SEPARATE SHEET OF PAPER Write the empirical formula for the following compounds 1 C 6H 6 2 C8H18 3 WO2 4 C2H6O2 5 X 39Y 13 6 A compound with an empirical formula of C2OH4 and a molar mass of 88 grams per mole What is the molecular formula of this compound 5 PRACTICE PROBLEM Using mass spectrometry the molecular weight of an organic compound was determined to be 84 068748 Identify whether the molecular formula of the compound is C 5 H 8 O C 4 H 8 N 2 or C 6 H 12 Use the following masses 1 H 1 007825 12 C 12 000000 14 N 14 003074 and 16 O 15 994915
Percent Composition and Molecular Formula Worksheet 1 What s the empirical formula of a molecule containing 65 5 carbon 5 5 hydrogen and 29 0 oxygen 2 If the molar mass of the compound in problem 1 is 110 grams mole what s the molecular formula This same approach may be taken considering a pair of molecules a dozen molecules or a mole of molecules etc The latter amount is most convenient and would simply involve the use of molar masses instead of atomic and formula masses as demonstrated Example 3 10 As long as the molecular or empirical formula of the compound in question is known the percent composition may be derived from
Molecular Formula Worksheet
https://s3.studylib.net/store/data/008992102_1-022f53585d7a7efb11e3a9f060aba7b9-768x994.png
Solved Empirical Molecular Formula Practice Worksheet Chegg
https://media.cheggcdn.com/study/06d/06d3e824-b032-432d-b735-42d1c4017b3a/image
Molecular Formula Worksheet - The molecular formula of a compound is defined as the actual number of atoms of elements covalently bonded in a molecule and is a whole number ratio of the empirical formula The percentage composition of a compound can be determined from its formula or from experimental mass proportion data Titanium oxide TiO2 is widely used as a pigment
