Identify Which Of The Following Equations Are Balanced To balance a chemical equation enter an equation of a chemical reaction and press the Balance button The balanced equation will appear above Use uppercase for the first character in the element and lowercase for the second character Examples Fe Au Co Br C O N F Ionic charges are not yet supported and will be ignored
Balance a chemical equation when given the unbalanced equation Explain the role of the Law of Conservation of Mass in a chemical reaction Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products Steps in Balancing a Chemical Equation Identify the most complex substance Beginning with that substance choose an element that appears in only one reactant and one product if possible Adjust the coefficients to obtain the same number of atoms of this element on both sides 3 1 1 to be interpreted in any of the following ways Two
Identify Which Of The Following Equations Are Balanced
Identify Which Of The Following Equations Are Balanced
http://www.sliderbase.com/images/referats/1120b/(11).PNG
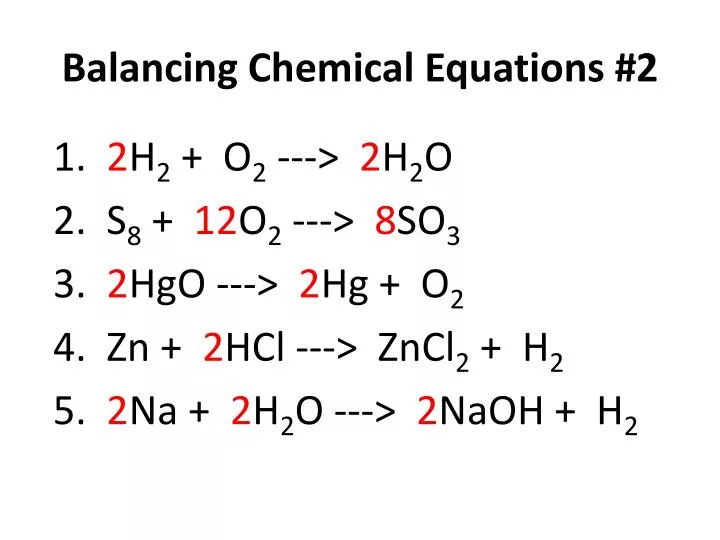
PPT Balancing Chemical Equations 2 PowerPoint Presentation Free
https://image2.slideserve.com/3871925/balancing-chemical-equations-2-n.jpg

Balanced Equations 2 YouTube
https://i.ytimg.com/vi/BEP9O52w3SE/maxresdefault.jpg
Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents The limiting reagent row will be highlighted in pink Examples of complete chemical equations to balance Fe Cl 2 FeCl 3 KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2 The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
We can balance it for charge by adding two electrons to the right side of the equation so that the net charge on each side is 0 Oxidation Ni s Ni A 2 a q 2 e Now that the oxidation half reaction is balanced it tells us that two electrons are produced for every atom of nickel oxidized But where do those electrons go Write balanced chemical equations for the reactions used to prepare each of the following compounds from the given starting material s In some cases additional reactants may be required solid ammonium nitrate from gaseous molecular nitrogen via a two step process first reduce the nitrogen to ammonia then neutralize the ammonia with an
More picture related to Identify Which Of The Following Equations Are Balanced

Which Of The Following Equations Is Balanced Brainly
https://us-static.z-dn.net/files/dce/276c07b2e2d7ce16b9f28df51be7a00b.jpg

How To Balance Chemical Equations YouTube
https://i.ytimg.com/vi/iUARzSxcKzk/maxresdefault.jpg

How To Balance Chemical Equations 10 Steps with Pictures
http://www.wikihow.com/images/b/b8/Balance-Chemical-Equations-Step-10Bullet1-Version-2.jpg
When we balance an equation we determine the ratio of reactants to products which allows for the total number of atoms of reactants to match the number of atoms of the products Since the type of atoms does not change nuclear processes are a different story and the number of atoms stays that same the total mass that goes into the chemical Part A Identify which of the following equations are balanced Check all that apply Mg s N 8 Mg N s CO g 2H 8 CH OH 1 2KCIO3 s 2KCI O2 g 2KMnO4 8 KMnO s MnO2 s O2 g Submit Request Answer Provide Feedback 4 while Part A Ca s Brz 1 CaBra s Express your answer as a chemical equation
Identify which of the following equations are balanced CO g 2H2 g CH3OH l 2KMnO4 s K2MnO4 s MnO2 s O2 g A chemical reaction is a process by which one or more substances transform into different substances via a chemical change Sometimes chemical reactions exhibit evidence that can be easily observed when they occur A molecular equation is sometimes simply called a balanced equation In a molecular equation any ionic compounds or acids are represented as neutral compounds using their chemical formulas The state of each substance is indicated in parentheses after the formula Huh Let s consider the reaction that occurs between AgNO 3 and NaCl
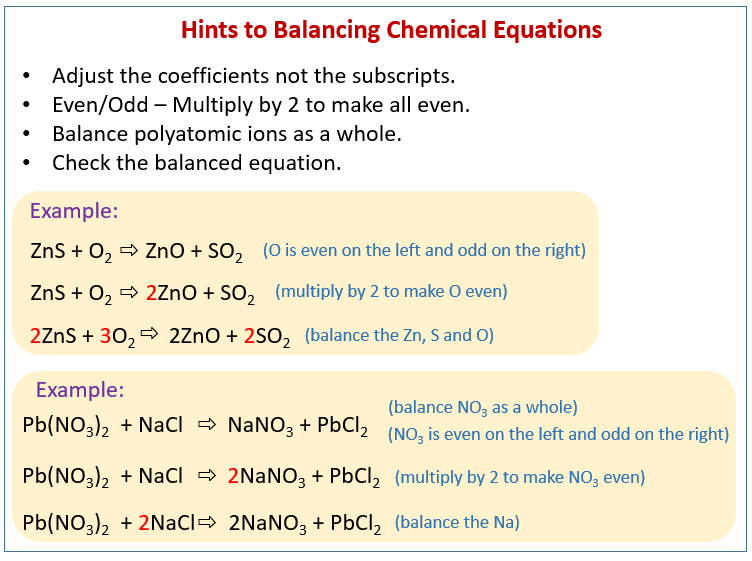
How To Balance Chemical Equations solutions Examples Videos
https://www.onlinemathlearning.com/image-files/xbalance-chemical-equations.png.pagespeed.ic.mtPzrx5_1V.png
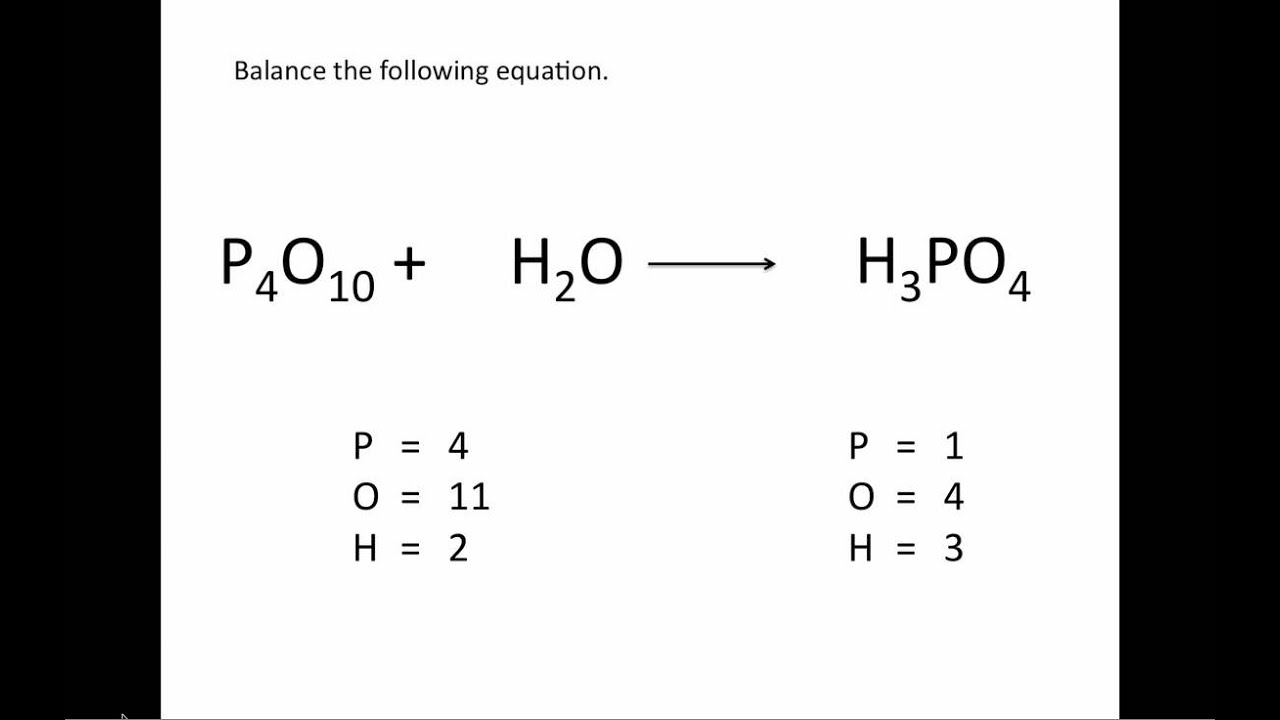
Balancing Chemical Equations UPDATED Chemistry Tutorial YouTube
https://i.ytimg.com/vi/HB6cG7bQew0/maxresdefault.jpg
Identify Which Of The Following Equations Are Balanced - Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents The limiting reagent row will be highlighted in pink Examples of complete chemical equations to balance Fe Cl 2 FeCl 3 KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2
.PNG)