Chapter 10 Chemical Reactions Answer Key 2 Count the number of atoms of the elements in the reactants 3 Count the number of atoms of the elements in the products 4 Change the coefficients to make the number of atoms of each element equal on both sides of the equation Write the coefficient in front of the formula never in the middle of a formula 5
Study Guide for Content Mastery Answer Key Matter and Change Created Date 12 11 2013 9 58 01 AM Answer potassium is a solid that is not diatomic bromine is a liquid that is diatomic potassium bromide s correct formula is KBr because K is 1 and Br is 1 in a compound so it is NOT KBr2 You never carry a formula over from the reactant side That is copy catting which is wrong
Chapter 10 Chemical Reactions Answer Key

Chapter 10 Chemical Reactions Answer Key
https://www.worksheeto.com/postpic/2012/11/types-of-chemical-reactions-worksheet-answer-key_243743.png
Types Of Chemical Reaction Worksheet Practice Answers
https://imgv2-1-f.scribdassets.com/img/document/345413836/original/bd72e78f72/1572596028?v=1
Fantastic Chapter 8 Chemistry Test Answers Chemical Equations And
https://s3.studylib.net/store/data/006622678_1-02100b34ecbf32b97080bb29573b033b.png
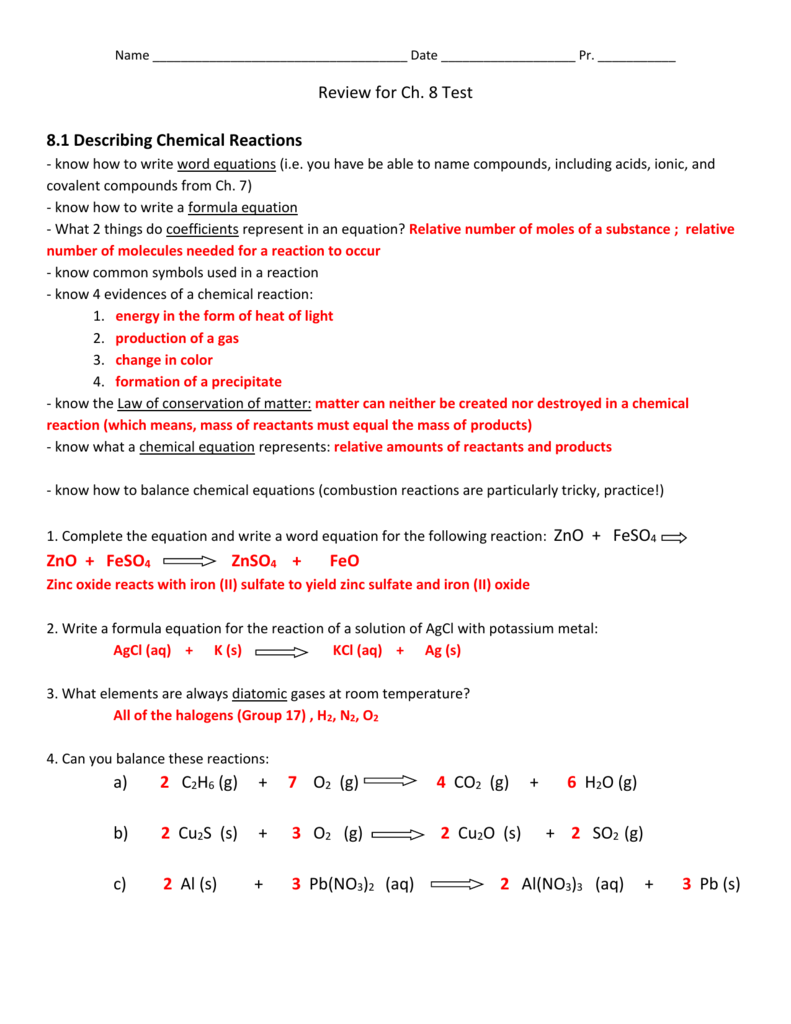
A n reaction results when ions between two ionic compounds interact to produce either a precipitate gas or water ion exchange A balanced chemical equation demonstrates the conservation of mass as are preserved in the reaction molecular weights an equal number of atoms Study with Quizlet and memorize flashcards containing Chapter 10 Chemical Reactions chemical reaction Click the card to flip The process by which the atoms of one or more substances is rearranged to form different substances Click the card to flip 1 32 Flashcards Learn Test Match Created by Lucile13 Teacher Terms in this set 32 chemical reaction
2 of chemical reaction Heat and light energy produced White ash produced 3 2 Mg s O2 g 2 MgO s 4 of chemical reaction Combination reaction B Zinc and Copper II Sulfate Time CuSO 4 aq Appearance Zn s Appearance Evidence of a Chemical Reaction initial Blue green Dull gray after 30 min Lt blue Black Change in color Acid Base Reactions An acid base reaction is one in which a hydrogen ion H is transferred from one chemical species to another Such reactions are of central importance to numerous natural and technological processes ranging from the chemical transformations that take place within cells and the lakes and oceans to the industrial scale production of fertilizers pharmaceuticals and other
More picture related to Chapter 10 Chemical Reactions Answer Key
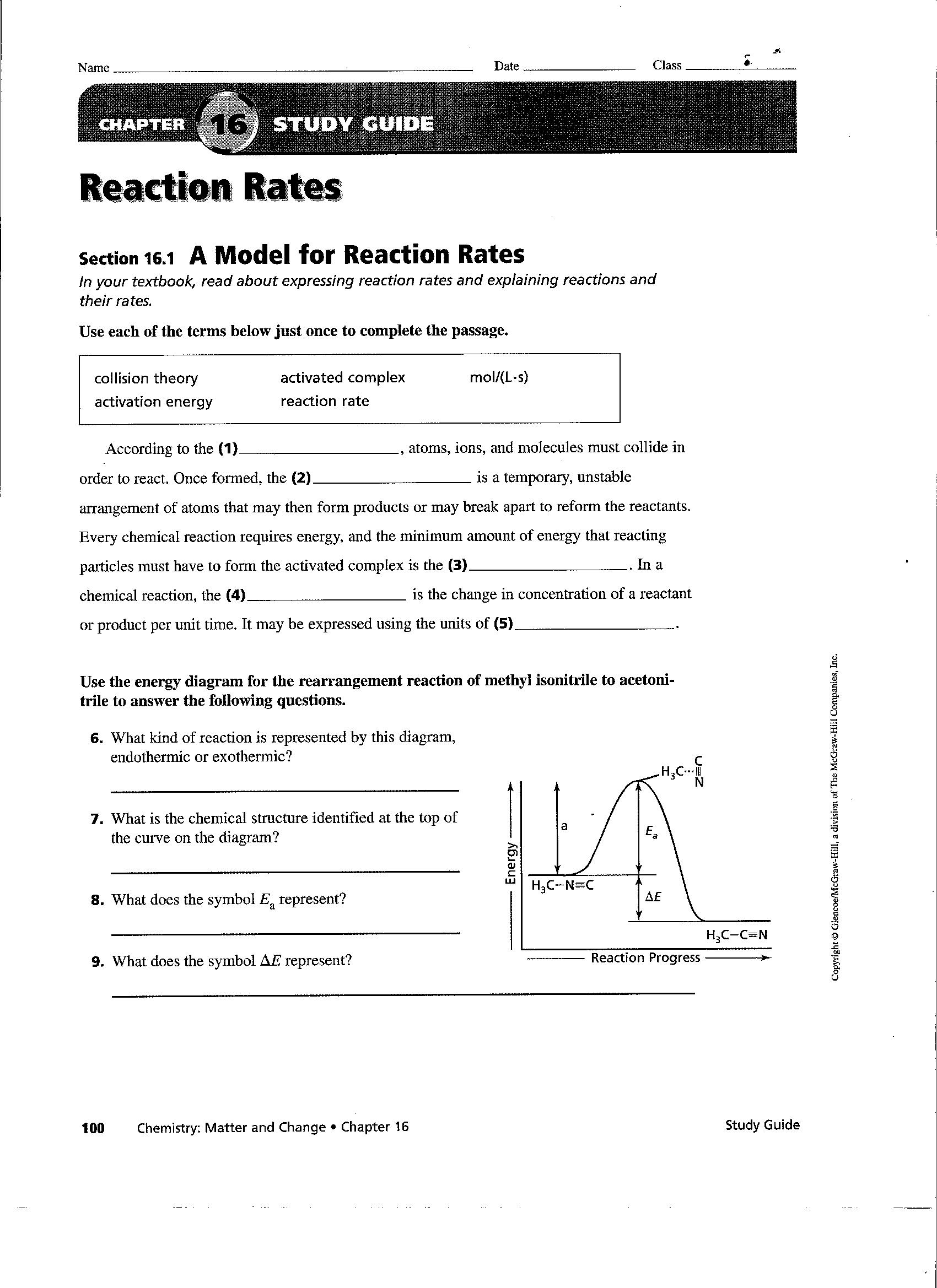
13 Worksheet Reaction Rates Answer Worksheeto
https://www.worksheeto.com/postpic/2012/04/chemistry-reaction-rates-worksheet-answers_240187.jpg

Chemical Reactions And Equations Worksheet Mcgraw Hill Tessshebaylo
https://media.cheggcdn.com/study/8a4/8a4532e0-017a-4c5d-8078-ba14f2e1a799/image.png

Chemistry Chapter 10 Chemical Reactions Study Guide Answers Study Poster
https://s3.studylib.net/store/data/009663771_1-cbd1af8980bc66678087c3fa16ba606c.png
Two moles of solid cesium react with 2 moles of liquid water to form 2 moles of aqueous cesium hydroxide and 1 mole of gaseous hydrogen These are homework exercises to accompany Chapter 10 of the University of Kentucky s LibreText for CHE 103 Chemistry for Allied Health Solutions are available below the questions Chemistry Chemical Reactivity 10th Edition Course Hero verified solutions and explanations Chapter 1 Basic Concept of Chemistry Chapter 2 Atoms Molecules and Ions Chapter 3 Chemical Reactions Chapter 4 Stoichiometry Quantitative Information about Chemical Reactions Chapter 5 Principles of Chemical Reactivity Energy and Chemical Reactions
A Hydrogen gas and iron chloride are produced b Chlorine gas and iron hydroxide are produced c No reaction takes place d Iron salt and water are produced Solution Hydrogen gas and iron chloride are produced Download NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations PDF Ans The process of decomposition of any molecule using electricity is termed as electrolytic decomposition The application of this is when we need to separate molecules which dissociate at high temperatures One such case would be sodium chloride Sodium Chloride dissociates at high temp but breaks apart easily on electrolytic decomposition 2

Classifying Chemical Reactions Worksheets Answer Key
https://www.unmisravle.com/wp-content/uploads/2018/06/classifying_chemical_reactions_worksheets_0.jpg

Types Of Chemical Reactions Worksheet Writing Formulas Answe
http://www.worksheeto.com/postpic/2012/11/types-of-chemical-reactions-worksheet-answer-key_243740.png
Chapter 10 Chemical Reactions Answer Key - In a chemical reaction matter is not created or destroyed but is conserved B matter can be created and destroyed but does not change forms C in a chemical reaction efforts should be made to preserve rare elements without changing them D in a chemical reaction the final mass of the products is always greater than the starting mass of
