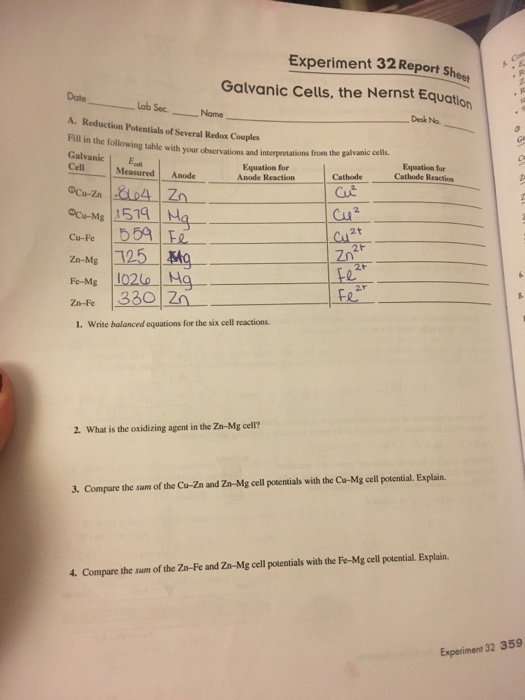
Experiment 32 Report Sheet Experiment 32 General Chemistry 2 Galvanic Cells Experiment 32 Galvanic Cells the Nernst Studocu Part A Reduction Potentials of Several Redox Couples Galvanic Cell Cathode positive Anode negative Cell Potential mV Cu Zn Cu Zn 0 Cu Pb Cu Pb 0 Cu Fe Cu Fe 0 Zn Pb Zn Pb 0 Fe Pb Fe Pb 0 Zn Fe Fe Zn 0 Data for Part A of experiment
General Chemistry II Lab CHEM 1310 Thursday s from 3 00 pm 5 45 pm Professor Constantino 26 April 2020 Experiment 32 Galvanic Cell the Nernst Equation Hypothesis If the relative reduction potentials for a number of redox couples are measured then it is possible to estimate the concentration of ions in a solution using the Nernst Equation The purpose of the lab was to measure the relative reduction potentials for a number of redox couples to develop an understanding of the movement of electrons anions and cations in a galvanic cell to study factors affecting cell potential to estimate the concentration of ions in solution using Nernst equation
Experiment 32 Report Sheet
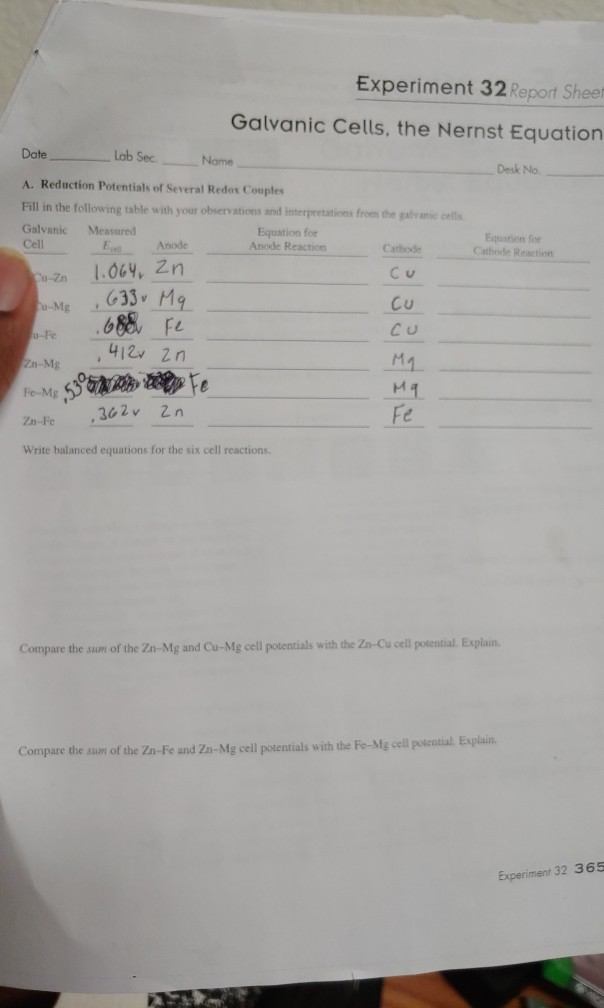
Experiment 32 Report Sheet
https://media.cheggcdn.com/media/681/6817d0c1-a150-4915-b0ee-5362c0bc19a4/image.png

Free Printable Lab Report Template Printable Templates
http://templatelab.com/wp-content/uploads/2017/08/lab-report-template-19.jpg?w=320

16 Exclusive Experiment Report Templates In MS WORD Writing Word
https://www.reportsexcel.com/wp-content/uploads/2022/05/experiment-report-template-855698705.jpg
Experiment 32 Galvanic Cells Background Information Standard Cell Potential is calculated as Eo cell Eo red Eo ox Values for the two half cell potentials are normally obtained from a table Pre Lab Hints 1 Refer to Figure 32 1 as well as the Chapter 19 class notes LEO and GER Applicable to redox systems that are not at standard conditions most often when the concentrations of the ions in solution are not 1 mol L Nernst equation at 25 C Ecell E cell 0 0592 n logQ n moles of electrons exchanged according to the cell reaction Q reaction quotient Q reaction quotient
Text Experiment 32 Report Sheet Galvanic Cells the Nernst Equation Date Lab Section Name Desk No Reduction Potentials Several Redox Couples Fill in the following table with our observations and interpretations from galvanic cells Experiment 32 Galvanic Cells Background Information Standard Cell Potential is calculated asEocell Eored E o ox Values for the two half cell potentials are normally obtained from a table Pre Lab Hints 1 Refer to Figure 32 1 as well as the Chapter 19 class notes LEO and GER
More picture related to Experiment 32 Report Sheet
18 Scientific Method Worksheet PDF Worksheeto
https://www.worksheeto.com/postpic/2013/02/science-experiment-lab-sheet-template_467796.png
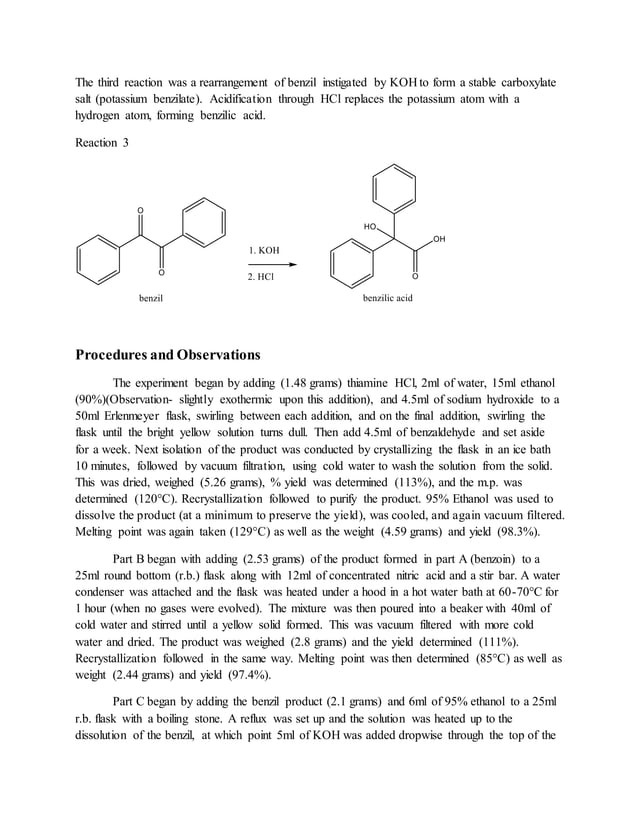
Experiment 32 Formal Report PDF
https://image.slidesharecdn.com/4c48caf7-2a65-4787-849e-0c7d24aefbdc-150416201750-conversion-gate02/85/experiment-32-formal-report-3-638.jpg?cb=1666685845
Solved Experiment 32 Report Sheet Galvanic Cells The Nernst Chegg
https://media.cheggcdn.com/study/9d9/9d9a6842-f124-44db-a37c-398a2eff359e/image
Experiment 32 Galvanic Cell the Nernst Equation Hypothesis In the use of several redox couples to determine Exp 32 Lab Report pdf Solutions Available Nova Southeastern University CHEM 1300 1310 lab Trending in CHEM 1310 Ayaan Khan Introduction to The Balance Sheet docxBDI docx York University BUSINESS 4613 VIDEO ANSWER We re told that copper half cell which serves as the cathode is used with some other half cell We have 14 aluminum iron and zinc and we re going to calculate the reduction potential for each Each component has an E cell for it
Experiment 32 Report Sheet Galvanic Cells the Nernst Equation Date Lab Sec Name Desk No A Reduction Potentials of Several Redox Couples Fill in the following table with your observations and interpretations from the galvanic cells Galvanic E cell Equation for Equation for Cell Download this page as a PDF Writing a Lab Report Return to Writing Studio Handouts Part 1 of 2 Introducing a Lab Report The introduction of a lab report states the objective of the experiment and provides the reader with background information State the topic of your report clearly and concisely in one or two sentences
Solved Experiment 32 Report Shee Galvanic Cells The Nernst Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/001/001c3a3b-741e-4e1f-baac-95daa55b7a58/image
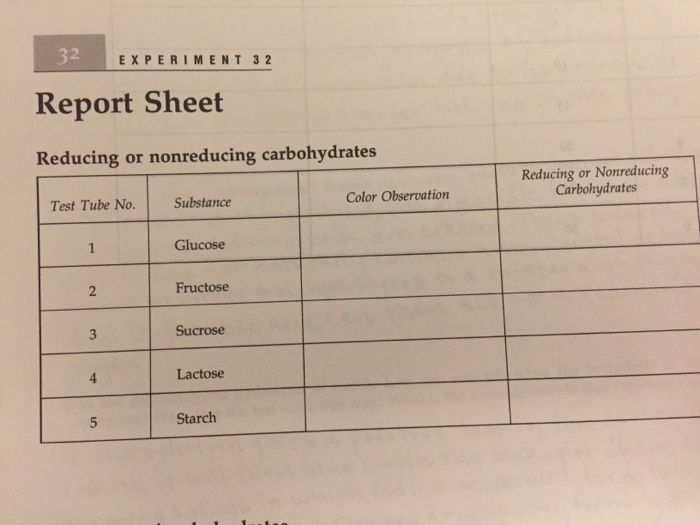
Solved 2 EXPERIMENT 32 Report Sheet Reducing Or Nonreducing Chegg
https://media.cheggcdn.com/media/c27/c2748f72-a99a-4836-a1cc-d491e6617a23/image.png
Experiment 32 Report Sheet - Experiment 32 Galvanic Cells Background Information Standard Cell Potential is calculated as Eo cell Eo red Eo ox Values for the two half cell potentials are normally obtained from a table Pre Lab Hints 1 Refer to Figure 32 1 as well as the Chapter 19 class notes LEO and GER