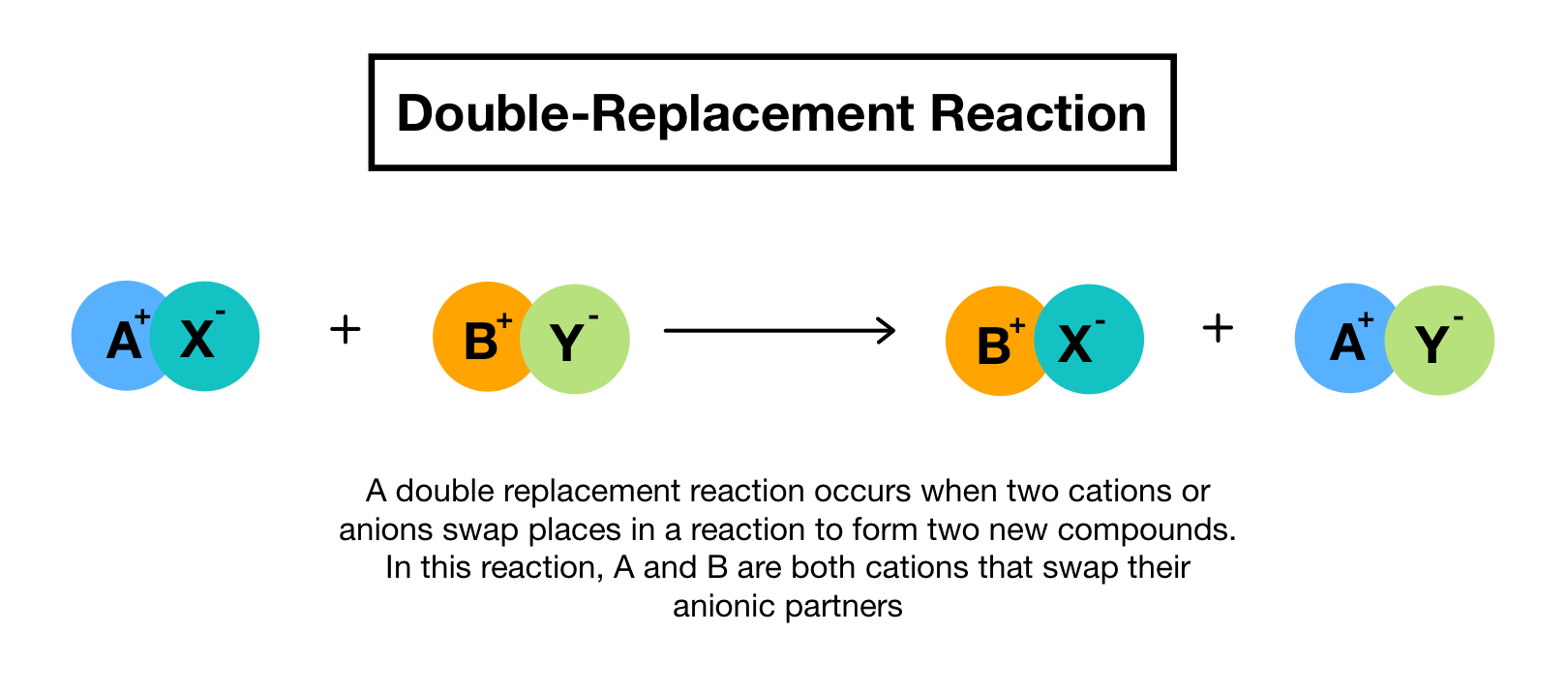
Double Replacement Reactions Worksheet Double replacement reactions also called double displacement exchange or metathesis reactions occur when parts of two ionic compounds are exchanged making two new compounds The overall pattern of a double replacement reaction looks like this A B C D A D C B
A double replacement reaction has the form AB CD AD CB There are four different possible outcomes to a reaction such as this 1 Formation of a gas There are certain compounds which are unstable and decompose to water and a gas Three common ones are H2CO3 H2SO3 and NH4OH They decompose like this A double replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds The general form of a double replacement also called double displacement reaction is ce AB ce CD rightarrow ce AD ce CB nonumber
Double Replacement Reactions Worksheet

Double Replacement Reactions Worksheet
https://s3.studylib.net/store/data/008707860_1-df3eea4a6f73f9467eb38fba77e85801.png

Chemistry Replacement Reaction Worksheet
https://i.pinimg.com/originals/4a/85/fc/4a85fc14bebd8bff07f09ea722fed418.png

Double Replacement Reaction Worksheet On Single Double Replacement
https://s3.studylib.net/store/data/006686627_1-ba579de56cc7d90859760f657966c018.png
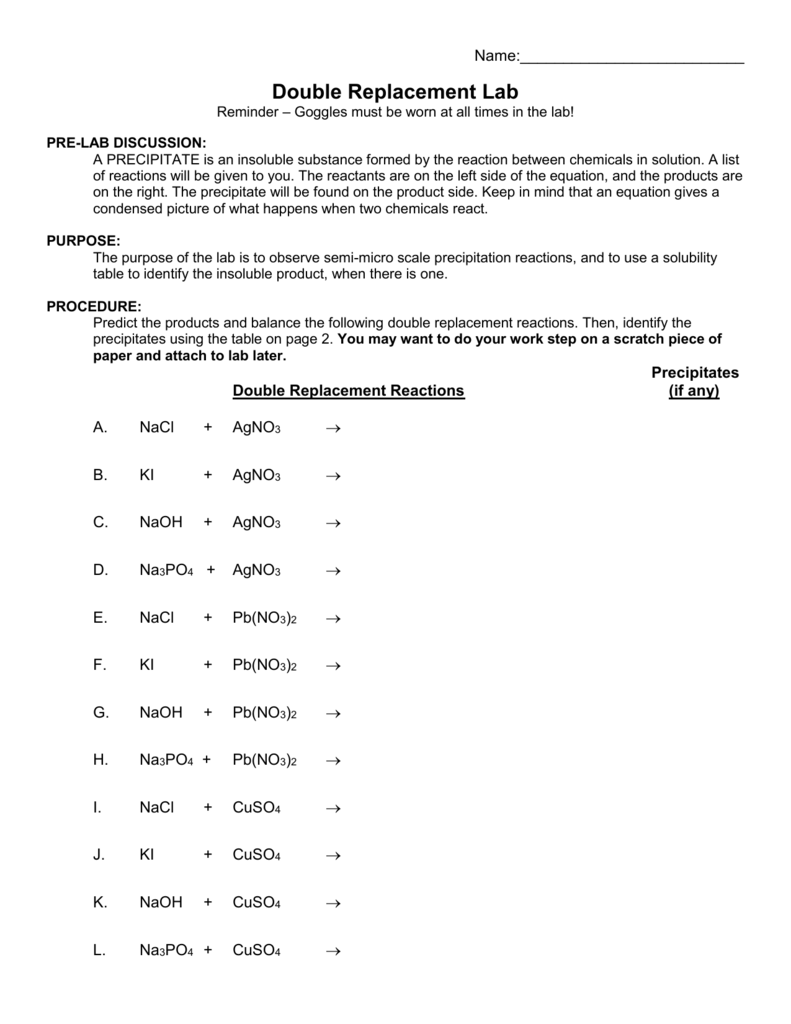
When a double replacement reaction occurs the cations and anions switch partners resulting in the formation of two new ionic compounds AD and CB one of which is in the solid state This solid product is an insoluble ionic compound called a precipitate To determine whether a product ionic compound will be soluble or insoluble consult the In a precipitation reaction one type of double replacement reaction two soluble ionic compounds in aqueous solution are mixed and result in an insoluble solid compound called a precipitate For example consider the reaction between aqueous lead II nitrate with aqueous potassium bromide as shown below Pb NO3 2 aq KBr aq
A single replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products Presented below 2HCl aq Zn s ZnCl2 aq H2 g 2 HCl aq Zn s ZnCl 2 aq H 2 g is an example of a single replacement reaction Double Replacement Reactions Worksheet Objectives Predict if a reaction will occur for double displacement rxns Predict the products of double displacement rxns Predict if these reactions will occur If yes complete and balance the equation You will need to use the Solubility Chart found in your book Double Replacement Reactions
More picture related to Double Replacement Reactions Worksheet
Double Replacement Reactions Definition Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/double-replacement_reaction_example.jpeg
Double Replacement Reaction Worksheet
https://s3.studylib.net/store/data/007019323_1-48844cbdaae7224f907e95d338191424.png

Double Replacement Reaction Worksheet
https://s3.studylib.net/store/data/008629482_1-9a4699a165c11885379b6c0915f2e263.png
A double replacement reaction also known as a double displacement reaction is a type of chemical reaction in which two reactants exchange ions to form two new compounds Double replacement reaction takes place in an aqueous solution and typically results in the formation of a precipitate Ionic compounds mostly participate in a double CHEMISTRY DOUBLE REPLACEMENT REACTION WORKSHEET AgNO3 NaCl AgCl NaNO3 CaCO3 HCl CaCl2 CO2 H2O 7 Pb NO3 2 CuSO4 PbSO4 Cu NO3 2 1 It is important that the formulas of the products be written correctly If they are correct balancing the equation is a simple task if not the equation will
During double replacement the cations and anions of two different compounds switch places Written using generic symbols it is AB XY AY XB A and X are the cations postively charged ions in this example with B and Y being the anions negatively charged ions Here is another way to look at the above generic example Double Displacement Reactions Solutions Indicate which of the following double displacement reactions will go to completion and circle what the product of the reaction will be if any You may want to consult a list of Ksp values to solve these problems 1 2 NaOH 1 CaBr2 2 NaBr 1 Ca OH 2 Will this reaction occur Yes 2 1 Pb NO3 2
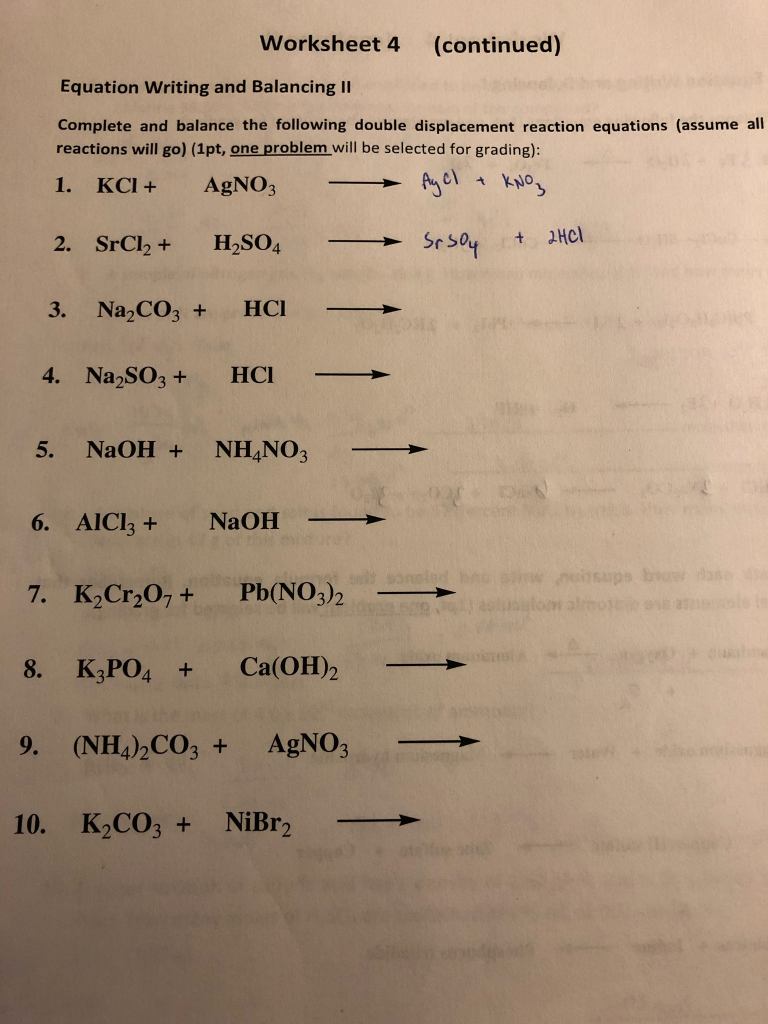
Double Replacement Reaction Worksheet
https://media.cheggcdn.com/media/2a9/2a9b7c30-0a8a-4ca8-9b50-ccd127990f83/php0JSujg.png
Double Replacement Reaction Worksheet
https://d2j6dbq0eux0bg.cloudfront.net/images/15443271/959670357.jpg
Double Replacement Reactions Worksheet - When a double replacement reaction occurs the cations and anions switch partners resulting in the formation of two new ionic compounds AD and CB one of which is in the solid state This solid product is an insoluble ionic compound called a precipitate To determine whether a product ionic compound will be soluble or insoluble consult the