Chemical Reactions And Equations Lab 10 Matter undergoes three kinds of change physical chemical and nuclear While the composition of a chemical substance is not altered by physical changes such as freezing and evaporation chemical changes or reactions result in the formation of new substances when bonds are formed and or broken Some relatively simple but common types of
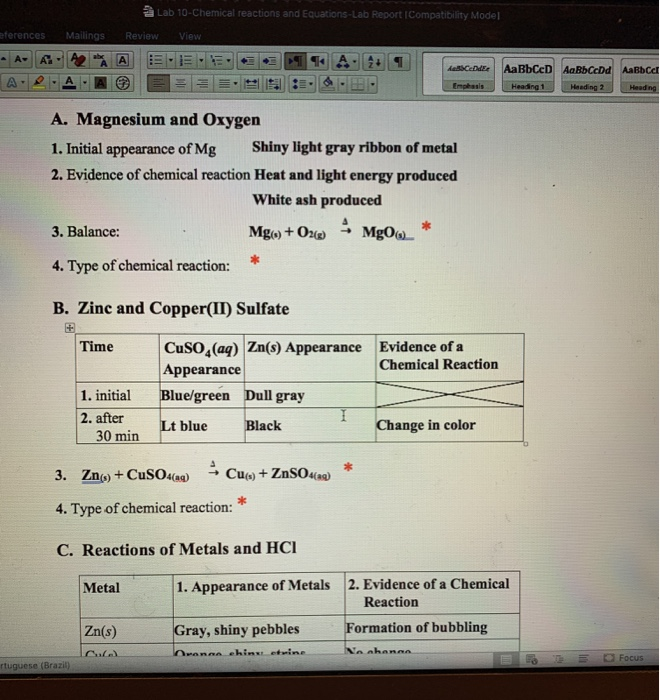
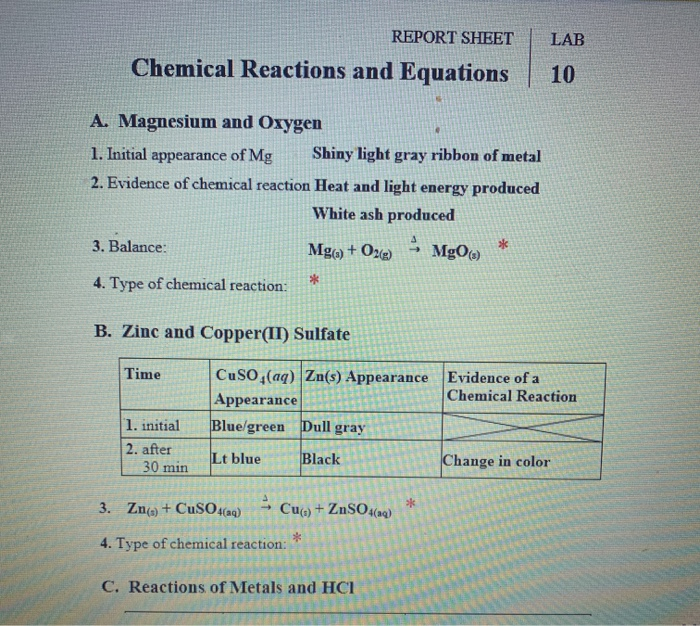
Perform Part I in your Kitchen Chemistry Lab Mix about 3mL of each solution in a clean and dry test tube Use pipettes to transfer solutions to test tubes You must write all equations for a given reaction before you start the next reaction Keep your goggles on You will be given the names of the compounds Chemistry questions and answers REPORT SHEET LAB Chemical Reactions and Equations 10 A Magnesium and Oxygen 1 Initial appearance of Mg Shiny light gray ribbon of metal 2 Evidence of chemical reaction Heat and light energy produced White ash produced 3 Balance Mg s O2 g MgO 4
Chemical Reactions And Equations Lab 10
Chemical Reactions And Equations Lab 10
https://media.cheggcdn.com/study/b84/b8487227-caac-4346-a4aa-3dc212796aee/image.png

Solved Chemical Reactions And Equations Report Sheet Lab 10 Chegg
https://media.cheggcdn.com/media/165/1651c4be-c2f7-4a6c-8f0e-4be6b94266c3/image.png

Solved Chemical Reactions And Equations Report Sheet Lab 10 Chegg
https://media.cheggcdn.com/media/dc0/dc0971a0-e209-44bd-bae3-d333b237e36c/image.png
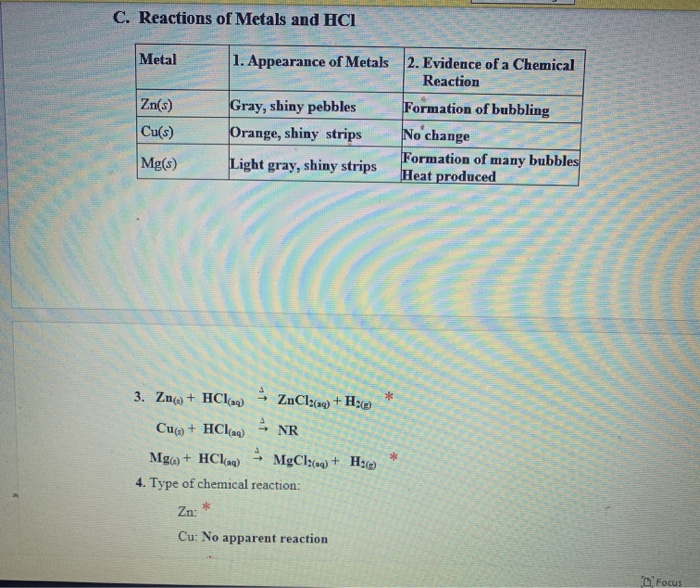
6 2 E Reactions of copper II sulfate solution and zinc 1 Add about 3 mL of 1 0 M copper II sulfate solution to a test tube Place a small amount of zinc metal in the solution Note the appearance of the solution and the zinc before and after the reaction The state of matter of reactants and products is designated with the symbols s for solids l for liquids and g for gases Chemical reaction a process in which one or more substances the reactants are converted to one or more different substances the products Substances are either chemical elements or compounds
8 Cabbage Chemistry In the Color changing Cabbage Chemistry activity students use cabbage to make an indicator solution and then learn about acids and bases by testing various foods and liquids 9 Foamy Fake Snow In the Foaming Fake Snow activity students make fake snow and explore chemical reactions and surfactants Heat over a Bunsen burner until the sulfur begins to burn Word equation Sulfur reacts with oxygen to yield sulfur dioxide gas Station 8 Place a small amount a small scoop of hydrated copper II sulfate into a small test tube Heat the hydrate over a Bunsen burner until the chemical reaction is complete the reaction should be obvious
More picture related to Chemical Reactions And Equations Lab 10
Solved REPORT SHEET LAB Chemical Reactions And Equations 10 Chegg
https://media.cheggcdn.com/study/1e5/1e50fd1a-b36e-4398-91c3-259292139786/image.png
Solved REPORT SHEET LAB Chemical Reactions And Equations 10 Chegg
https://media.cheggcdn.com/study/d02/d02e6869-6dfe-4934-ab00-e4a82e8daf46/image.png

Types Of Chemical Reactions Pogil Answer Key Six Types Of Chemical
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/b67335e04c559b9fda33bf8f3d7c29e2/thumb_1200_1553.png
Chemical Reactions and Equations Hypothesis Prior to this lab we learned the different types of reactions how to balance equations and the importance of balancing equations In this lab we will do different experiments and get their reaction predict the products of the reactions and then classify the reaction I predict that most if not Each side of the reaction has two chromium atoms seven oxygen atoms two nitrogen atoms and eight hydrogen atoms In a balanced chemical equation both the numbers of each type of atom and the total charge are the same on both sides Equations 4 1 1 and 4 1 2 are balanced chemical equations What is different on each side of the equation is
In an equation for a chemical reaction the reactants are written on the and products are written on the seperates two or more formulas what the plus sign do reacts to form products what does the arrow do reactants are heated what does the arrow with triangle above mean solid what does s mean Study with Quizlet and memorize flashcards containing terms like Determine whether each observation generally corresponds to a physical change or a chemical change a Bubbles are produced upon mixing two solutions b A precipitate is formed from two solutions c A liquid freezes into a solid d A solution heats up upon mixing with another e A solid dissolves into water f The color of a

Chemical Reactions And Equations Class 10 Science CBSE NCERT KVS YouTube
https://i.ytimg.com/vi/43G7DnxgJCI/maxresdefault.jpg

NCERT Class 10th Science Chapter 1 Chemical Reactions And Equations
https://i.ytimg.com/vi/ZAP5c5vorLY/maxresdefault.jpg
Chemical Reactions And Equations Lab 10 - Heat over a Bunsen burner until the sulfur begins to burn Word equation Sulfur reacts with oxygen to yield sulfur dioxide gas Station 8 Place a small amount a small scoop of hydrated copper II sulfate into a small test tube Heat the hydrate over a Bunsen burner until the chemical reaction is complete the reaction should be obvious