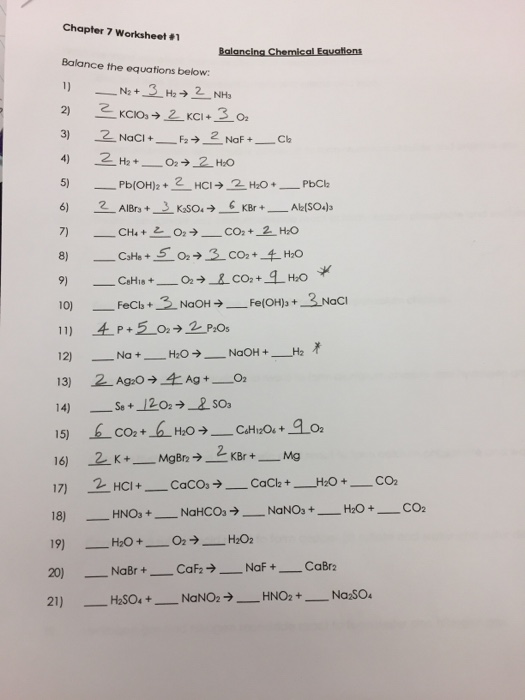Challenge Questions Balance The Equations Below BALANCED CHEMICAL EQUATION A balanced equation is an equation in which the number of atoms of each element on both sides of the equation To balance a chemical equation we make use of coefficients which are numbers placed in front of elements or compounds The balanced form of the following equations using coefficients are 2NaNO3 PbO
Balancing Equations Challenge Part A Parts Pieces 1 Circle each subscript in each chemical formula 2 Draw a square around each coefficient 3 Answer the questions related to each chemical formula O 2 CO 2 5H 2 What element does the O represent How many atoms of each element are in the formula shown Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions
Challenge Questions Balance The Equations Below
Challenge Questions Balance The Equations Below
https://media.cheggcdn.com/media/11f/11fe337d-6786-4ebb-a5d7-6042737d4dc4/image

Balancing Chemical Equations Practice Worksheets With Answer
http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-36.jpg
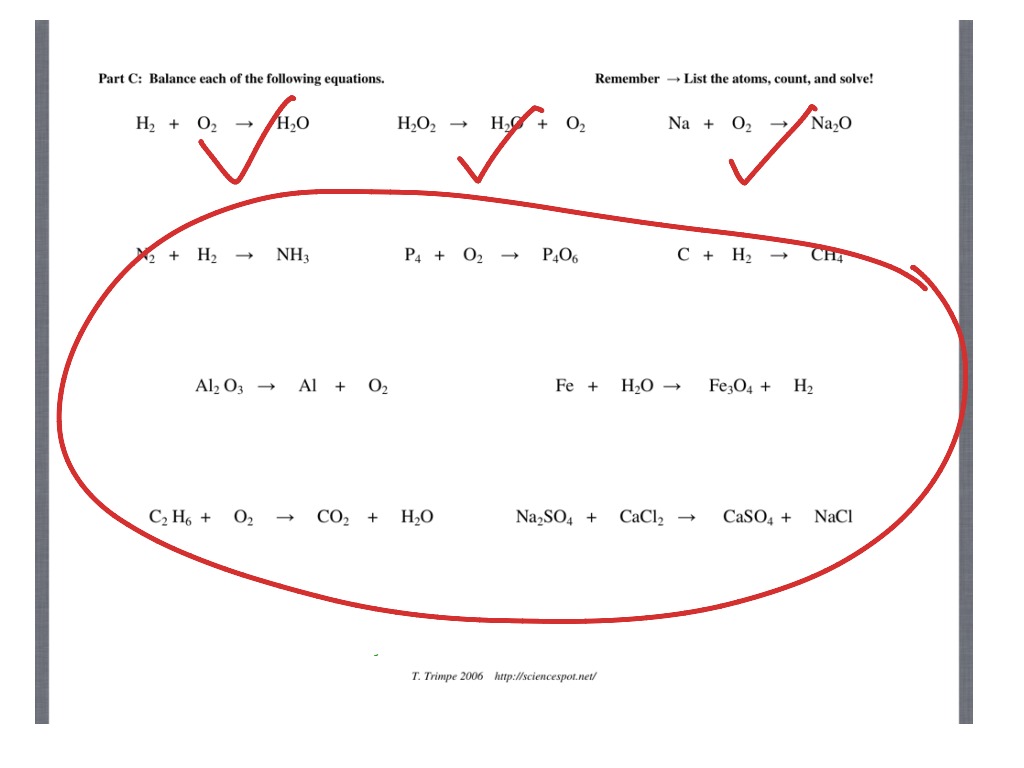
Balancing Equations Practice Worksheet Answers
https://showme0-9071.kxcdn.com/files/414777/pictures/thumbs/793258/last_thumb1363657451.jpg
A balanced equation is an equation where both sides are equal to the same amount The answer to the expression on the left side of the equals sign should be equal to the value on the right side of the equals sign In an unbalanced equation either the left hand side of the equation has a greater value than the right hand side like the example below Good job You completed the quiz so you got practice balancing equations However you missed some questions so you might want to review the steps to balancing equations or print free practice worksheets If you feel ready to move on learn about mass relations in balanced equations Are you ready to try another quiz
Explain your answer 7 As a group play level 1 of the balancing equation game Write down the strategies your group uses to balance chemical equations 8 Start level 2 of the balancing equation game Take turns in your group to balance the equations in the sim using your strategies from Level 1 and adding new strategies as needed 5 2 10 2 5 10 10 10 yes The numbers of N and O atoms on either side of the equation are now equal and so the equation is balanced Exercise 4 1 1 4 1 1 Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen molecular oxygen and water
More picture related to Challenge Questions Balance The Equations Below

More Balancing Equations Worksheet Answers
https://i.pinimg.com/originals/34/5b/cd/345bcdc5533e2956a67188650e537f5b.jpg
![]()
Equations Worksheet Practice Questions Cazoomy
https://cdn.shortpixel.ai/client/q_glossy,ret_img,w_510,h_718/http://www.cazoomy.com/questions/equations-questions-3.png

Balancing Equation Worksheet With Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-25.jpg
Tips for Balancing Equations When balancing equations remember chemical reactions must satisfy conservation of mass Check your work to make certain you have the same number and type of atoms on the reactants side as on the products side A coefficient number in front of a chemical is multiplied by all the atoms in that chemical CHALLENGE QUESTIONS BALANCE THE EQUATIONS BELOW A NaNO3 PbO Pb NO3 2 Nazo B Ca3P2 H2O Ca OH 2 PH3 C Fe2O3 CO Fe CO2 D NH3 02 NO2 H20 E FeS 02 Fe2O3 SO2 F C3H602 02 CO2 H20 This problem has been solved
N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have Simply putting her to the equation is balanced now coming to the second equation Here we have 3 calcium here 1 calcium So let us put here 3 now here we have 1 plus 2 pope 1 phosphorus Let us here to phosphor calcium became balanced now coming hydrogen here 32632 z 6 point so 6 Hydrogen means 126 plus 612 means

49 Balancing Chemical Equations Worksheets with Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-04.jpg

Balance Chemical Equations Worksheet
https://sciencenotes.org/wp-content/uploads/2015/01/balanceequations3-768x994.png
Challenge Questions Balance The Equations Below - Explain your answer 7 As a group play level 1 of the balancing equation game Write down the strategies your group uses to balance chemical equations 8 Start level 2 of the balancing equation game Take turns in your group to balance the equations in the sim using your strategies from Level 1 and adding new strategies as needed