Balancing Equations Race Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions
Balancing Equations Race 1 C3H8 02 CO2 H2O 2 AI FesNz AIN Fe 3 Na CI2 NaCI 4 H202 H2O O2 5 C6H12O6 02 H2O CO2 6 H2O CO2 C7H8 O2 7 NaClOa NaCI O2 8 NH4 3P04 Pb N03 4 Pb3 P04 4 NH4NO3 9 BF3 Li2S03 62 803 3 LiF 10 C7H17 02 CO2 H2O 11 CaCOg H3PO4 Ca3 P04 2 H2CO3 How do you know if a chemical equation is balanced What can you change to balance an equation Play a game to test your ideas
Balancing Equations Race

Balancing Equations Race
https://db-excel.com/wp-content/uploads/2019/09/balancing-chemical-equations-worksheet-1-answers-3-768x996.jpg

Dlewis Blog Notes On Kinetics And Balancing Equations FOR
https://4.bp.blogspot.com/-cXMknVLJeSo/T5VQWPs86oI/AAAAAAAAA5k/Molrep32sjw/s1600/intro+to+reactions_22.jpeg

Balancing Equations Help Sheet Teaching Resources
https://d1e4pidl3fu268.cloudfront.net/f36bf922-c388-43ea-abf1-9ee255cd6f38/FRONTCOVERFreebie.crop_958x720_0,0.preview.jpg
Balancing Chemical Equations PhET Interactive Simulations Welcome to It s Elemental Balancing Act Welcome to It s Elemental Balancing Act The computer will give you a number of incomplete chemical equations Balance the chemical equations by selecting coefficients from the pull down menus Once you think the equation is balanced press the Check my answer button Have fun and good luck
Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems Balancing Equations Race Solutions 1 1 C3H8 5 O2 3 CO2 4 H2O 2 2 Al 1 Fe3N2 2 AlN 3 Fe 3 2 Na 1 Cl2 2 NaCl 4 2 H2O2 2 H2O 1 O2 5 1 C6H12O6 6 O2 6 H2O 6 CO2 6 4 H2O 7 CO2 1 C7H8 9 O2 7 2 NaClO3 2 NaCl 3 O2 8 4 NH4 3PO4 3 Pb NO3 4 1 Pb3 PO4 4 12 NH4NO3
More picture related to Balancing Equations Race

Balancing Equations Worksheet 1 Answer Key Equations Worksheets
https://i0.wp.com/www.unmisravle.com/wp-content/uploads/2018/04/balancing_equations_practice_worksheet_answers_2.jpg
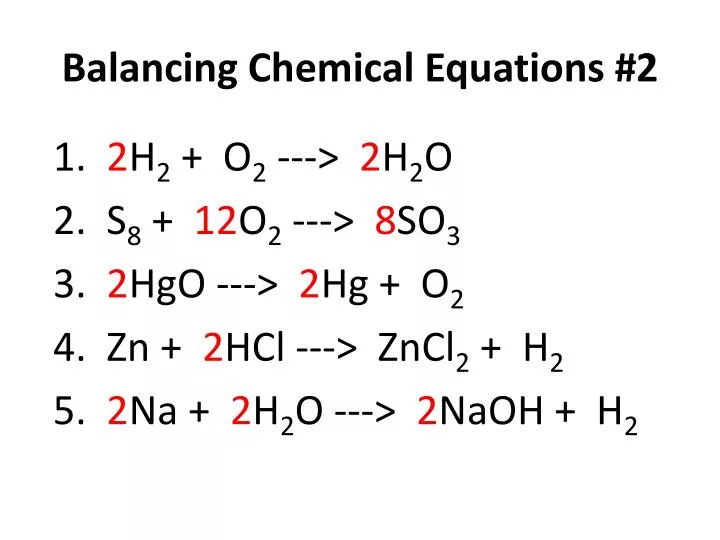
PPT Balancing Chemical Equations 2 PowerPoint Presentation Free
https://image2.slideserve.com/3871925/balancing-chemical-equations-2-n.jpg

Ck 12 Balancing Equations Answer Key 13 Best Images Of Practice
https://excelguider.com/wp-content/uploads/2019/07/worksheet-balancing-equations-practice-worksheet-balancing-throughout-balancing-chemical-equations-worksheet-1-answer-key-768x1056.jpg
Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the equation and only Gabrielle M 9 years ago I m working on Chemical Reactions Double and Single Replacement on FLVS Now my first question for this video is how do you have 4 aluminum atoms when it says 2Al subscript 2 Do you just add the 2 and the subscript 2 Same thing goes with the O3 I am so lost
Make sure that all coefficients are in the lowest possible ratio If necessary reduce to the lowest ratio Example 11 3 1 11 3 1 Balancing Chemical Equations Aqueous solutions of lead II nitrate and sodium chloride are mixed The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead II chloride Balancing Equations The chemical equation described above in Figure PageIndex 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter

Solved Balancing Equations Race 9 CsHg 02 CO2 Chegg
https://media.cheggcdn.com/study/c5b/c5bad468-275f-4c51-9be8-0fbe963255c2/image.png

Balancing Equations Race Worksheet Equations Worksheets
https://www.equationsworksheets.net/wp-content/uploads/2022/03/balancing-equations-race-worksheet-answers-db-excel.png
Balancing Equations Race - Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems