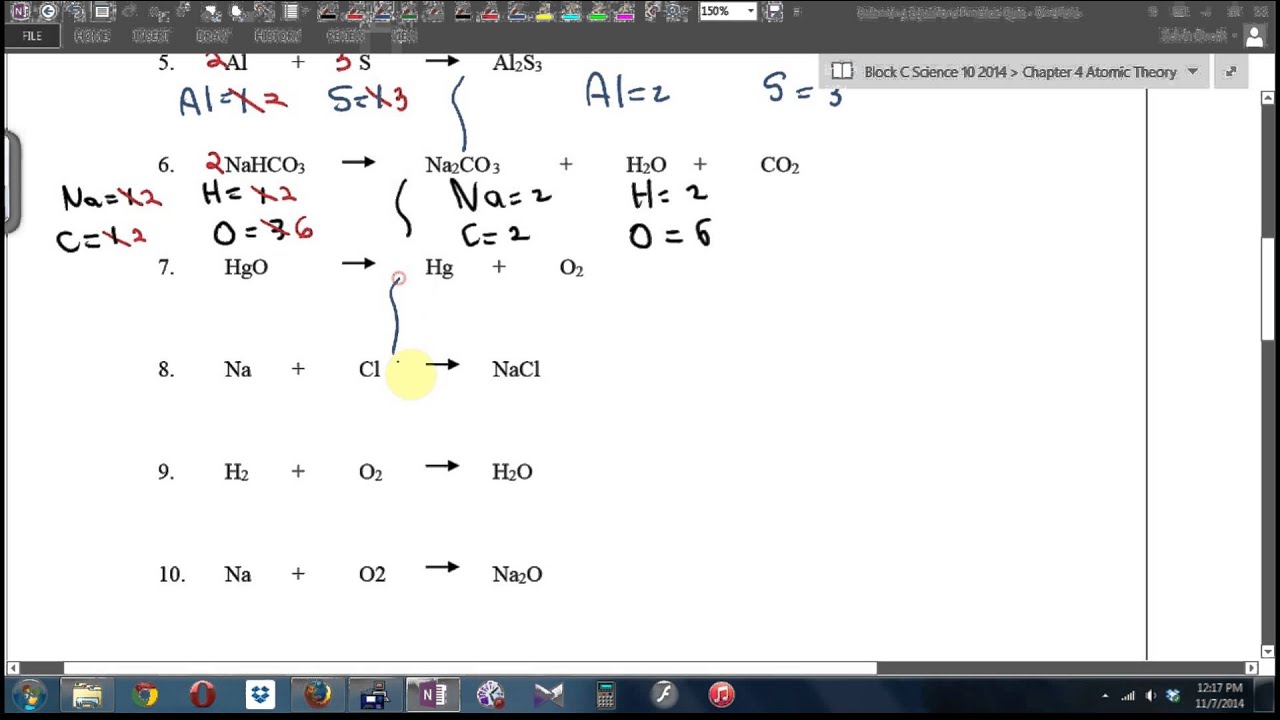
Balancing Equations Race Answer Key Balancing Equations Race Solutions 1 1 C3H8 5 O2 3 CO2 4 H2O 2 2 Al 1 Fe3N2 2 AlN 3 Fe 3 2 Na 1 Cl2 2 NaCl 4 2 H2O2 2 H2O 1 O2 5 1 C6H12O6 6 O2 6 H2O 6 CO2 6 4 H2O 7 CO2 1 C7H8 9 O2 7 2 NaClO3 2 NaCl 3 O2 8 4 NH4 3PO4 3 Pb NO3 4 1 Pb3 PO4 4 12 NH4NO3
Balancing Equations Race 1 C3H8 02 CO2 H2O 2 AI FesNz AIN Fe 3 Na CI2 NaCI 4 H202 H2O O2 5 C6H12O6 02 H2O CO2 6 H2O CO2 C7H8 O2 7 NaClOa NaCI O2 8 NH4 3P04 Pb N03 4 Pb3 P04 4 NH4NO3 9 BF3 Li2S03 62 803 3 LiF 10 C7H17 02 CO2 H2O 11 CaCOg H3PO4 Ca3 P04 2 H2CO3 Updated on January 04 2019 A balanced chemical equation gives the number and type of atoms participating in a reaction the reactants products and direction of the reaction Balancing an unbalanced equation is mostly a matter of making certain mass and charge are balanced on the reactants and products side of the reaction arrow
Balancing Equations Race Answer Key

Balancing Equations Race Answer Key
https://www.worksheeto.com/postpic/2012/01/balancing-chemical-equations-worksheet-answer-key_329456.png

Chemistry Balancing Chemical Equations Worksheet Answer Key Pdf
https://i0.wp.com/db-excel.com/wp-content/uploads/2019/09/balancing-chemical-equations-worksheet-answers-110-705x970.jpg

Foothill High School Balancing Chemical Equations Ii Answers Db excel
https://db-excel.com/wp-content/uploads/2019/09/foothill-high-school-balancing-chemical-equations-ii-answers.jpg
An answer key is provided Balancing Equations Online pdf and Balancing Equations Online 2 pdf Two different versions are available for this internet lesson about balancing equations Links for students can be found in the Sites for Students area Snowman Challenge Game Chal lenge your students to a game of balancing equations Print Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions
PROBLEM 5 1 1 7 5 1 1 7 A novel process for obtaining magnesium from sea water involves several reactions Write a balanced chemical equation for each step of the process The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide The second step is the formation of Balancing Chemical Equations Answer Key Balance the equations below N2 3 H2 2 NH3 KClO3 2 KCl 3 O2 2 NaCl 1 F2 2 NaF 1 Cl2 2 H2 1 O2 2 H2O Pb OH 2 2 HCl 2 H2O 1 PbCl2 AlBr3 3 K2SO4 6 KBr 1 Al2 SO4 3 CH4 2 O2 1 CO2 2 H2O
More picture related to Balancing Equations Race Answer Key

49 Balancing Chemical Equations Worksheets with Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-04.jpg
A Balancing Act Worksheet Answers Key Balancing Act Worksheet Answer
https://i.ytimg.com/vi/BiWmXY_xpEc/maxresdefault.jpg
Spice Of Lyfe Grade 10 Balancing Chemical Equations Worksheet
https://lh6.googleusercontent.com/proxy/R5dgvLgG2YS4lULaLTBqnHH3OcHknDT9rBJIAbsXd9DzHbfJ_KaITlovr_nJ9sdde1w9cW0aFGcBKZCkkPpepWCUap_HvqEWeHL6_HvgVT6VdqLm01uqll_2Qom--aL-=s0-d
N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems
In the case of this reaction where you have 2 in front of H and H O the 2 s mean that the reaction involves two molecules of H and two molecules of H O Since there s no number in front of O we assume that the coefficient is 1 and that there s one molecule of it involved in this reaction Updated 3 2 23 Balancing equations worksheets Balancing equations practice sheet Let s balance some equations Balancing equations practice sheet 2 More balancing Balancing Equations II dd ch Practice balancing Balancing Equations III dd ch More practice balancing Balancing Equations IV dd ch Still more practice balancing

Balancing Equations Race Answers Chemfiesta Tessshebaylo
https://s3.studylib.net/store/data/008265142_1-bb2b75a0f00d26f0856221bd15f2cf3f.png

Solved Balancing Equations Race 9 CsHg 02 CO2 Chegg
https://media.cheggcdn.com/study/c5b/c5bad468-275f-4c51-9be8-0fbe963255c2/image.png
Balancing Equations Race Answer Key - PROBLEM 5 1 1 7 5 1 1 7 A novel process for obtaining magnesium from sea water involves several reactions Write a balanced chemical equation for each step of the process The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide The second step is the formation of