Balancing Chemical Equations Balance The Equations Below Balance Chemical Equation Online Balancer Instructions on balancing chemical equations Enter an equation of a chemical reaction and click Balance The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character
Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions How do you know if a chemical equation is balanced What can you change to balance an equation Play a game to test your ideas
Balancing Chemical Equations Balance The Equations Below
Balancing Chemical Equations Balance The Equations Below
https://d2vlcm61l7u1fs.cloudfront.net/media/166/166c7328-9ce9-4fe3-b2ce-5e59e5e30ef8/image

Article Jackipaper web fc2
http://1217986664s505.typepad.com/files/equationsws.jpg

How To Balance Chemical Equations 10 Steps with Pictures
http://www.wikihow.com/images/b/b8/Balance-Chemical-Equations-Step-10Bullet1-Version-2.jpg
Balance a chemical equation when given the unbalanced equation Explain the role of the Law of Conservation of Mass in a chemical reaction Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products Al O O Al Al O O O I don t know if I m doing it right or not I am completely lost If someone can help me I would really appreciate it Thanks 4 comments
To balance a chemical equation you add these whole number multipliers coefficients to make sure that there are the same number of atoms on each side of the arrow Here s something important to remember about coefficients they apply to every part of a product For instance take the chemical equation for water H2O 11 Chemical Reactions
More picture related to Balancing Chemical Equations Balance The Equations Below

Balancing Chemical Equations Chapter 7 Worksheet 1 Printable Word
https://i2.wp.com/www.worksheeto.com/postpic/2012/01/balancing-chemical-equations-worksheet-1-answers_329462.png
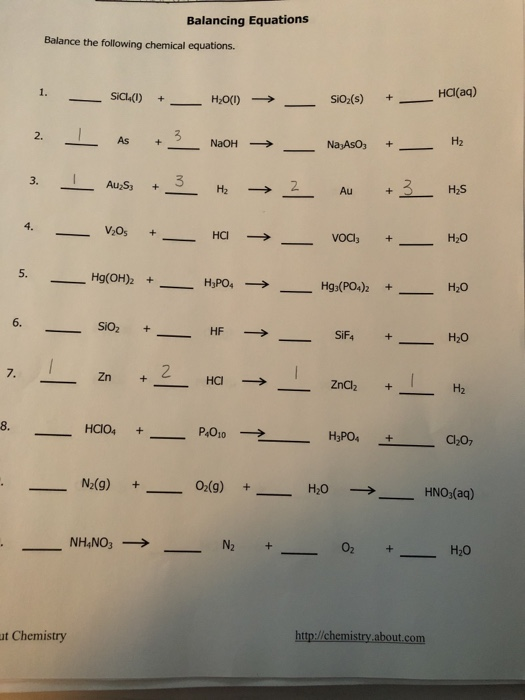
Solved Balancing Equations Balance The Following Chemical Chegg
https://media.cheggcdn.com/media/b9d/b9dddfc2-7df1-47ea-8244-b5ee0a8a528f/image.png

Practice Balancing Equations Answer Key Balancing Chemical Equations
https://briefencounters.ca/wp-content/uploads/2018/11/balancing-chemical-equations-worksheet-answer-key-and-balancing-chemical-equations-worksheet-with-answers-grade-10-luxury-of-balancing-chemical-equations-worksheet-answer-key-677x1024.jpg
The chemical equation for this reaction above would be written as 2 H 2 g O 2 g 2 H 2 O g This form of the chemical equation is called a balanced chemical equation A balanced chemical equation is one that conforms to the law of conservation of matter Balancing chemical equations 1 Google Classroom Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems
The chemical formula of propane is C 3 H 8 It burns with oxygen O 2 to form carbon dioxide CO 2 and water H 2 O The unbalanced chemical equation can be written as C3H8 O2 CO2 H2O Step 2 The total number of atoms of each element on the reactant side and the product side must be compared The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
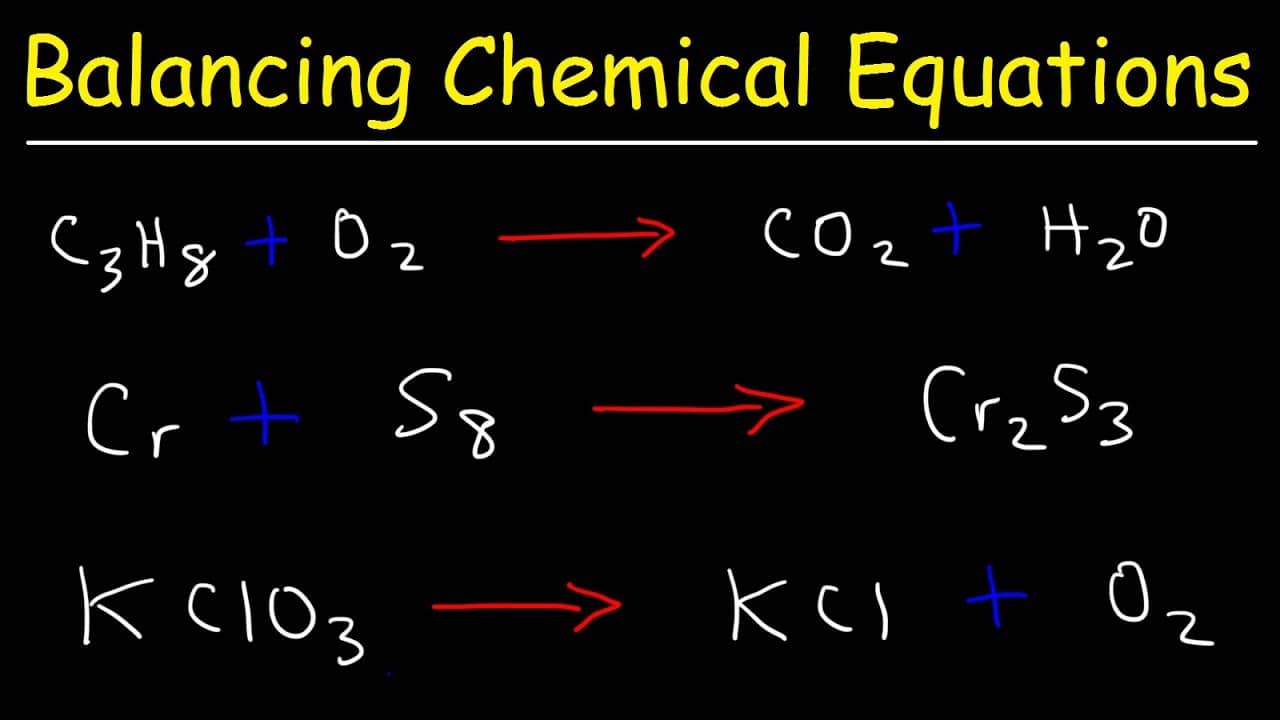
How To Balance Chemical Equations Best Examples Get Education Bee
https://geteducationbee.com/wp-content/uploads/2020/11/How-to-Balance-Chemical-Equations.jpg

Unique Unbalanced Equation Examples Modern Physics Class 12 Pdf
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-07.jpg
Balancing Chemical Equations Balance The Equations Below - Method 1 Doing a Traditional Balance Download Article 1 Write down your given equation For this example you will use C 3 H 8 O 2 H 2 O CO 2 This reaction occurs when propane C 3 H 8 is burned in the presence of oxygen to produce water and carbon dioxide 2 Write down the number of atoms per element
