8 07 Quiz How To Balance A Chemical Equation What does a chemical equation describe Click the card to flip a chemical reaction Click the card to flip 1 3 Flashcards Learn Test Match Q Chat Created by Ocean0307 Students also viewed k12 PS chemical reaction 8 01 8 08 13 terms FEW ANNALU5 Preview ahhh science unit 8 11 terms kyliec2295 Preview 9 01 Quiz Acids and Bases sarahwolff16
Balancing Chemical Equations 4 4 48 reviews CaO H O Ca OH Click the card to flip balanced Click the card to flip 1 31 Flashcards Test Match Q Chat Created by ChemIsTree4U Students also viewed World War I Teacher 23 terms Christine Evans52 Preview Balancing Chemical Equations 40 terms alnmo6 Preview This online quiz is intended to give you extra practice in balancing identifying and predicting a random selection of over 150 chemical equations This quiz aligns with the following NGSS standard s HS PS1 2 HS PS1 7 Select your preferences below and click Start to give it a try
8 07 Quiz How To Balance A Chemical Equation

8 07 Quiz How To Balance A Chemical Equation
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-08.jpg
How To Balance Chemical Equations Best Examples Get Education Bee
https://geteducationbee.com/wp-content/uploads/2020/11/How-to-Balance-Chemical-Equations.jpg
How To Balance Chemical Equations solutions Examples Videos
https://www.onlinemathlearning.com/image-files/xbalance-chemical-equations.png.pagespeed.ic.mtPzrx5_1V.png
Take a balancing chemical equations quiz to gain confidence in chemistry Here is a ten question balancing chemical equations quiz Each question presents an unbalanced equation Select the balanced equation Find the answer key below the questions Remember the number of each type of atoms is the same on both sides of the reaction arrow when 1 Choose the correctly balanced equation for the following reaction Potassium water potassium hydroxide hydrogen gas 2K 2H O 2KOH H K H O KOH 2H 2K 2H O K HO K
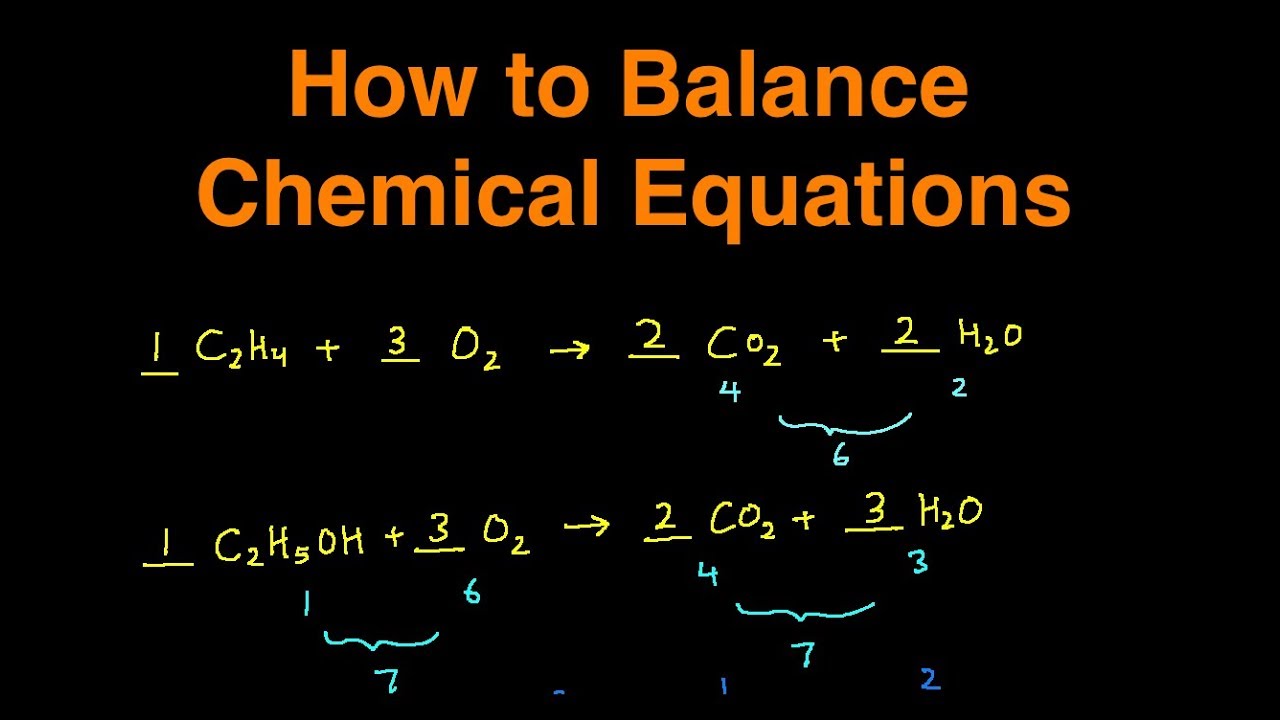
Balancing chemical equations 1 Google Classroom Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have N2 H2 2NH3 Total the atoms up again On the left there is 2 N and 2 H still On the right there is 2 N and 6 H now So now all we need to do is make the left side have 6 H in total
More picture related to 8 07 Quiz How To Balance A Chemical Equation
How To Balance Chemical Equations Step By Step Explanation With
https://i.ytimg.com/vi/mxSQBLsN3-Y/maxresdefault.jpg

Balance Chemical Equations Worksheet
https://sciencenotes.org/wp-content/uploads/2015/01/balanceequations3-768x994.png

How To Balance Chemical Equations 11 Steps with Pictures Chemical
https://i.pinimg.com/originals/e5/e0/98/e5e098839c6638360d6e6172461b7b16.jpg
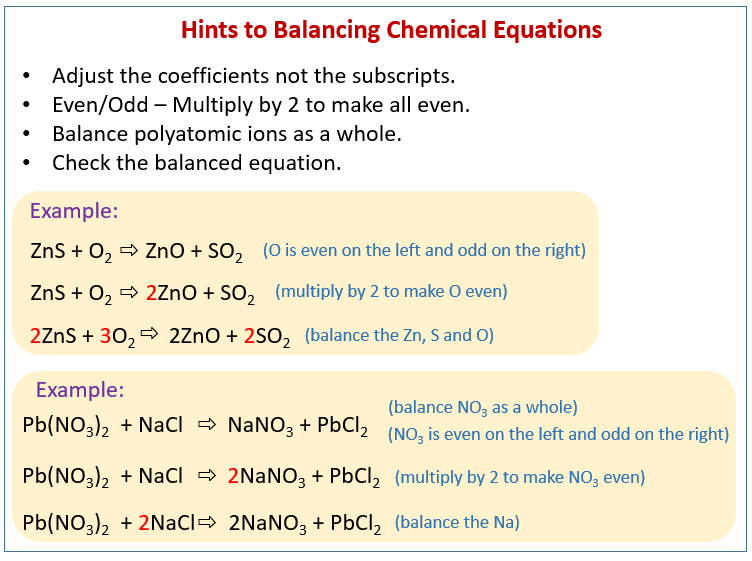
The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed To balance a chemical equation you add these whole number multipliers coefficients to make sure that there are the same number of atoms on each side of the arrow Here s something important to remember about coefficients they apply to every part of a product For instance take the chemical equation for water H2O
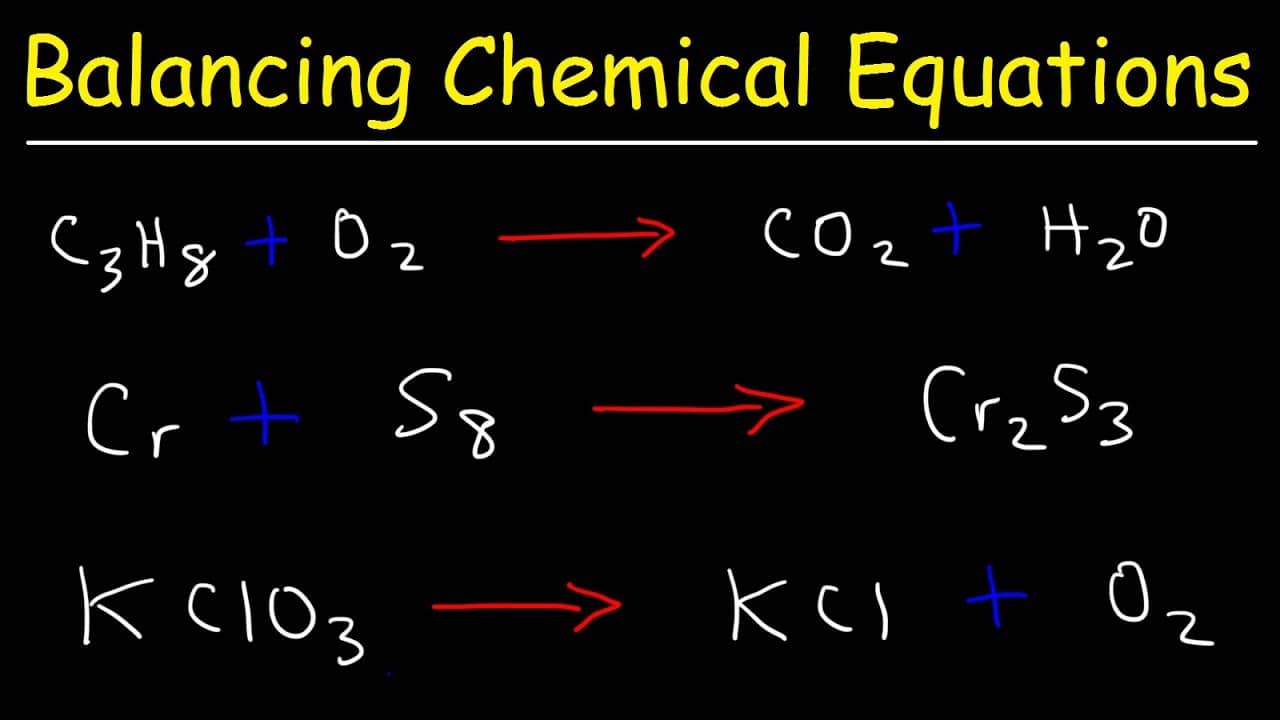
Step 1 The unbalanced equation must be obtained from the chemical formulae of the reactants and the products if it is not already provided The chemical formula of propane is C 3 H 8 It burns with oxygen O 2 to form carbon dioxide CO 2 and water H 2 O The unbalanced chemical equation can be written as C3H8 O2 CO2 H2O Step 2 Follow four easy steps to balance a chemical equation Write the unbalanced equation to show the reactants and products Write down how many atoms of each element there are on each side of the reaction arrow Add coefficients the numbers in front of the formulas so the number of atoms of each element is the same on both sides of the equation

How To Balance Chemical Equations 10 Steps with Pictures
http://www.wikihow.com/images/b/b8/Balance-Chemical-Equations-Step-10Bullet1-Version-2.jpg

49 Balancing Chemical Equations Worksheets with Answers
http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-26.jpg?w=320
8 07 Quiz How To Balance A Chemical Equation - Method 1 Doing a Traditional Balance Download Article 1 Write down your given equation For this example you will use C 3 H 8 O 2 H 2 O CO 2 This reaction occurs when propane C 3 H 8 is burned in the presence of oxygen to produce water and carbon dioxide 2 Write down the number of atoms per element