Stoichiometry Practice Answer Key Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction
Answers 1A 30 mol Ag 1B 30 mol AgNO3 1C 20 mol H2O 1D 10 mol NO 2A 38 mol N2H4 2B 19 mol N2O4 2C 76 mol H2O 3 191 g Al2O3 B How many moles of aluminum oxide are made if 3580 g of manganomanganic oxide are consumed C How many moles of manganomanganic oxide will react with 5 33 x 1025 atoms of aluminum D Practice Problems Stoichiometry Answer Key Balance the following chemical reactions a 2 CO O 2 2 CO 2 b 2 KNO 3 2 KNO 2 O 2 c 2 O 3 3 O 2 d NH 4 NO 3 N 2 O 2 H 2 O e 4 CH 3 NH 2 9 O 2 4 CO 2 10 H 2 O 2 N 2 f Cr OH 3 3 HClO 4 Cr ClO 4 3 3 H 2 O Write the balanced chemical equations of each reaction a
Stoichiometry Practice Answer Key

Stoichiometry Practice Answer Key
https://i.pinimg.com/originals/e7/99/0b/e7990b8ad502dabd90a5477f6445bb9e.png

Stoichiometry Practice Problems Worksheet Answers Pdf
https://i2.wp.com/images.sampletemplates.com/wp-content/uploads/2016/11/17162152/Gas-Stoichiometry-Worksheet.jpg
Stoichiometry Worksheet Answers
https://imgv2-1-f.scribdassets.com/img/document/131383825/original/a396110907/1521550172?v=1
Google Classroom You might need Calculator Periodic table Given the following reaction Zn CuCl A 2 ZnCl A 2 Cu How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess moles round to three significant figures Show Calculator Show Periodic Table Stuck Stoichiometry questions Google Classroom One type of anaerobic respiration converts glucose C 6 H 12 O 6 to ethanol C 2 H 5 O H and carbon dioxide If the molecular weight of glucose is 180 grams mol and the molar mass of ethanol is 46 g mol how many grams of carbon dioxide are produced when 1 mol of glucose is digested via respiration
Question 10 Identify the energy graphs A and B as being for an endothermic or exothermic reaction A B Answer 4 7 Unit 4 Practice Problems is shared under a not declared license and was authored remixed and or curated by LibreTexts KEY Solutions for the Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N2 3 F2 2 NF3 2 2 C6H10 17 O2 12 CO2 10 H2O 3 4 5 1 HBr 1 KHCO3 1 H2O 1 KBr 1 CO2 2 GaBr3 3 Na2SO3 1 Ga2 SO3 3 6 NaBr 3 SnO 2 NF3 3 SnF2 1 N2O3
More picture related to Stoichiometry Practice Answer Key

Stoichiometry Worksheet With Answer Key Printable Pdf Download
https://data.formsbank.com/pdf_docs_html/73/732/73239/page_1_thumb_big.png

Stoichiometry Worksheet Answer Key
https://briefencounters.ca/wp-content/uploads/2018/11/stoichiometry-worksheet-answer-key-also-stoichiometry-gizmo-worksheet-answers-fresh-quiz-amp-worksheet-of-stoichiometry-worksheet-answer-key.jpg

Basic Stoichiometry Phet Lab Answer Key Reactants Products And
https://www.wsfcs.k12.nc.us/cms/lib/NC01001395/Centricity/Domain/6847/Exam Review Pg 2101112014_0000.jpg
Introduction You might use stoichiometry skills to double a cookie recipe Image credit Chocolate Chip Cookies by Kimberley Vardeman on Wikimedia Commons CC BY 2 0 A balanced chemical equation is analogous to a recipe for chocolate chip cookies It shows what reactants the ingredients combine to form what products the cookies Homework Set 1 1 2C3H7OH 9O2 6CO2 8H2O a How many moles of O2 are required to react with 58 moles of C3H7OH b How many moles of CO2 are required to react with 17 moles of O2 c How many grams of CO2 would be required to react with 7 8 moles of H2O d How many grams of C3H7OH are needed to produce 0 45 moles of water
Stoichiometry with Solutions Problems Stoichiometry with Solutions Name 1 H3PO4 3 NaOH Na3PO4 3 H2O How much 0 20 M H3PO4 is needed to react with 100 ml of 0 10 M NaOH 2 2 HCl Zn ZnCl2 H2 When you use 25 ml of 4 0 M HCl to produce H2 gas how many grams of zinc does it react with This online quiz is intended to give you extra practice in performing stoichiometric conversions including limiting reagent and percent yield problems This quiz aligns with the following NGSS standard s HS PS1 7 Select your preferences below and click Start to give it a try Number of problems 15102550 Chemical equations are Balanced

Stoichiometry Is Easy Chemical Education Xchange
https://www.chemedx.org/sites/www.chemedx.org/files/images/article/stoichiometry-easy.jpg
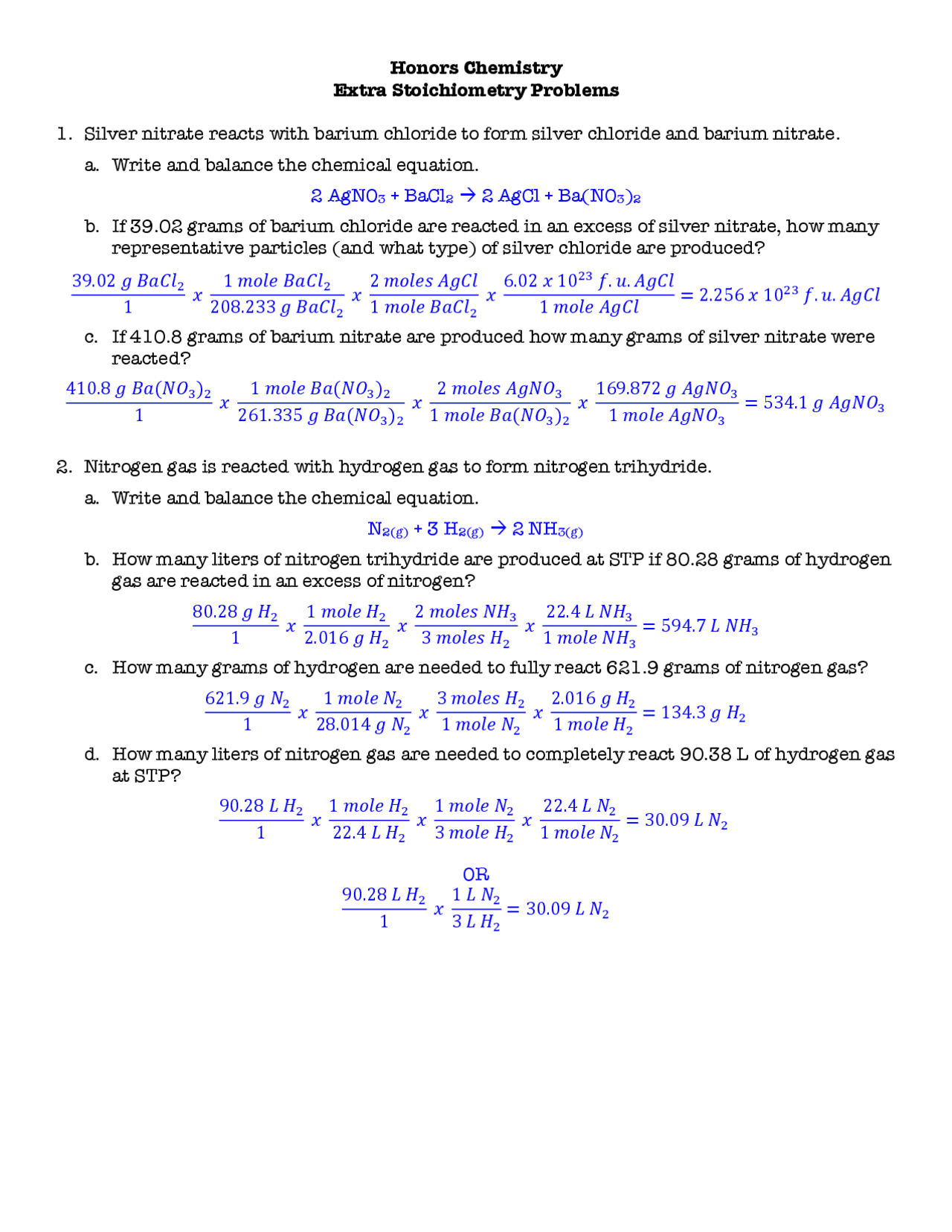
41 Chemfiesta Stoichiometry Practice Worksheet Answers Worksheet Master
https://static.docsity.com/documents_first_pages/2021/04/20/374813fc83f05cecfbdbe5809326f2b0.png
Stoichiometry Practice Answer Key - KEY Solutions for the Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N2 3 F2 2 NF3 2 2 C6H10 17 O2 12 CO2 10 H2O 3 4 5 1 HBr 1 KHCO3 1 H2O 1 KBr 1 CO2 2 GaBr3 3 Na2SO3 1 Ga2 SO3 3 6 NaBr 3 SnO 2 NF3 3 SnF2 1 N2O3
