Writing Formula Equations Ws 1 Created by Marrinator91 Teacher Step 1 Write each formula and balance each formula using SUBSCRIPTS Step 2 Balance the overall equation using COEFFICIENTS Terms in this set 12 sulfur S oxygen sulfur dioxide S 8 O 8 SO zinc sulfuric acid H SO zinc sulfate hydrogen Zn H SO ZnSO H
Here s the best way to solve it Expert verified View the full answer Previous question Next question Transcribed image text Class period Date Name Unit Chemical Reactions Writing Formula Equations WS 1 Directions Convert the following word equations into formula equations by changing element and compound names into chemical formulas 1 Worksheet 1 Writing and Balancing Formula Equations Step 1 Write each formula and balance each formula using SUBSCRIPTS Step 2 Balance the overall equation using coefficients 1 sulfur 2 zinc 3 hydrogen nitrogen ammonia 4 hydrogen chlorine hydrogen chloride 5 carbon 6 calcium oxide 7 phosphorus 8 hydrochloric acid 9 barium
Writing Formula Equations Ws 1

Writing Formula Equations Ws 1
https://cdn.slidesharecdn.com/ss_thumbnails/equationswritingws3-090627112616-phpapp01-thumbnail-4.jpg?cb=1246101997
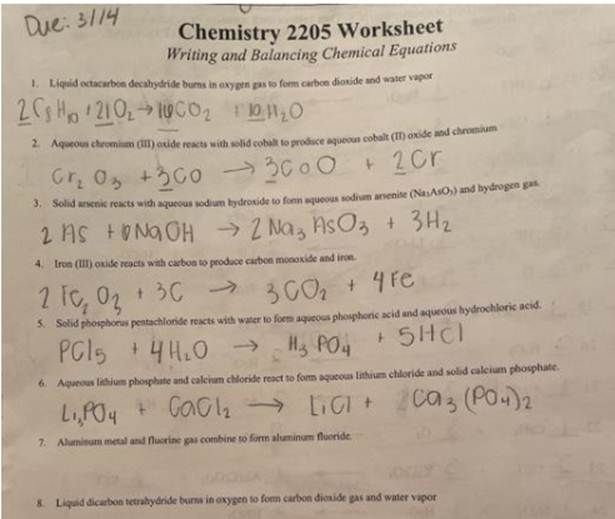
Get Answer Due 3114 Chemistry 2205 Worksheet Writing And Balancing
https://files.transtutors.com/book/qimg/21cc2d83-3d2e-431e-b7ed-1ae3f17d6e5a.png
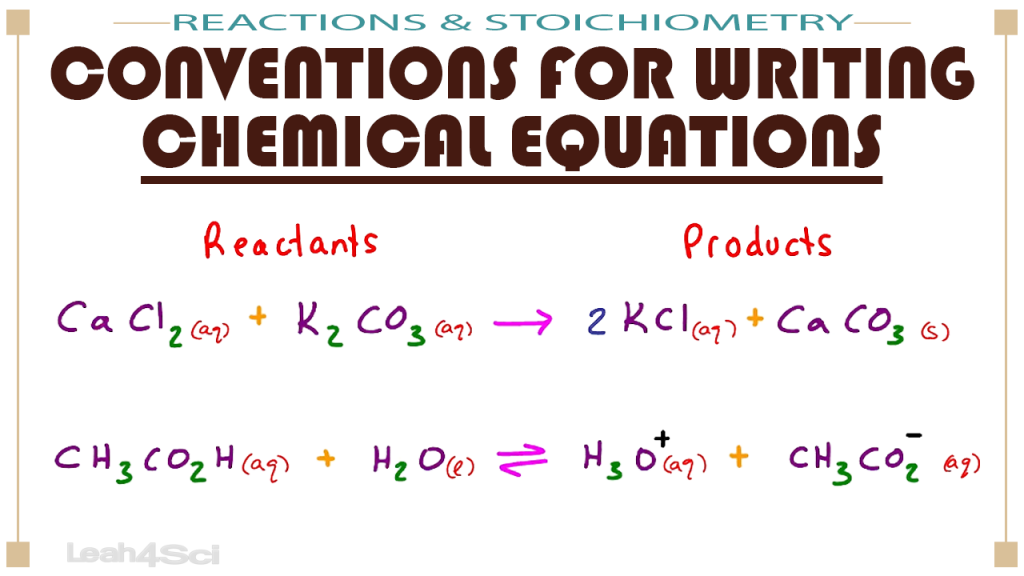
Conventions For Writing Chemical Equations
https://leah4sci.com/wp-content/uploads/2019/06/Conventions-for-Writing-Chemical-Equations-MCAT-General-Chemistry-by-Leah4sci-1024x576.png
Note that the number of atoms for a given element is calculated by multiplying the coefficient of any formula containing that element by the element s subscript in the formula If an element appears in more than one formula on a given side of the equation the number of atoms represented in each must be computed and then added together Worksheet Writing and Balancing Formula Equations Step 1 Write each formula and balance each formula using SUBSCRIPTS
Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules A conventional balanced equation with integer only coefficients is derived by multiplying each coefficient by 2 2C2H6 7O2 6H2O 4CO2 5 1 5 5 1 5 2 C 2 H 6 7 O 2 6 H 2 O 4 CO 2 Finally with regard to balanced equations recall that convention dictates use of the smallest whole number coefficients
More picture related to Writing Formula Equations Ws 1

Writing Complete Equations Practice
https://s3.studylib.net/store/data/008265152_1-66faf7613fffb441a9dd131a07d6e879.png

PPT Ch 8 Chemical Equations And Reactions PowerPoint Presentation
https://image2.slideserve.com/4293738/writing-equations-l.jpg

Equations Writing Ws 1
https://cdn.slidesharecdn.com/ss_thumbnails/equationswritingws1-090627112625-phpapp02-thumbnail-4.jpg?cb=1246102008
Results for Unit Chemical Reactions Writing Formula Equations WS1 TPT Results for Unit Chemical Reactions Writing Formula Equations WS1 17 000 results Sort by Relevance View List Describing Chemical Reactions Using Equations Worksheet Created by Good Science Worksheets NaCl aq H 2O l electricity NaOH aq H 2 g Cl 2 g Write balanced molecular complete ionic and net ionic equations for this process Answer Chemical equations are symbolic representations of chemical and physical changes Formulas for the substances undergoing the change reactants and substances generated by the change products
1 NaBr sodium bromide 2 CaO calcium oxide 3 Li 2 S lithium sulfide 4 MgBr 2 magnesium bromide 5 Be OH 2 beryllium hydroxide Write the formulas for the following ionic compounds 6 potassium iodide KI 7 magnesium oxide MgO 8 aluminum chloride AlCl 3 9 sodium nitrate NaNO 3 10 calcium carbonate CaCO 3 1 15 Flashcards Learn Test Match Q Chat Created by g cejalol Terms in this set 15 A chemical equation represent a chemical reaction Law of Conservation of Atoms There must be the same number of each type of atom before the reaction as after the reaction Synthesis Two elements combine to form a compound E E C Decomposition

Writing Formula Equations Assignment
https://s3.studylib.net/store/data/007062236_1-8bf2ca46d6bc67956c24f5675a592669-768x994.png
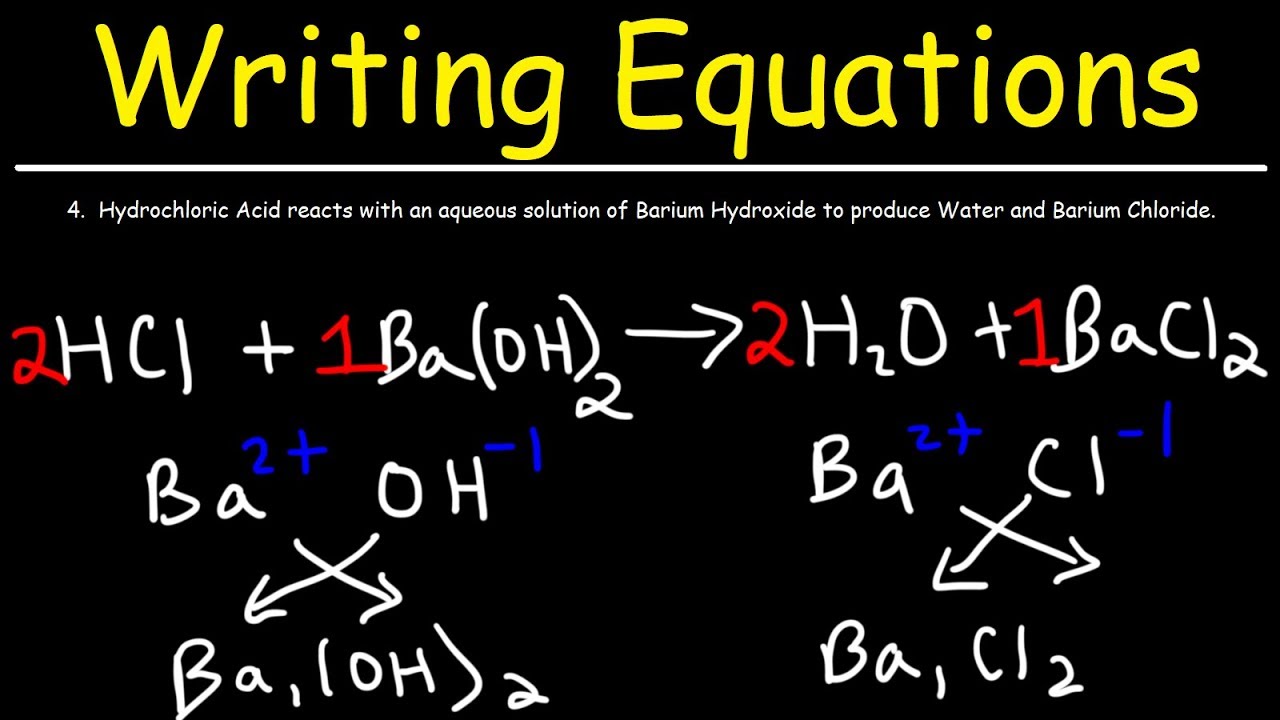
How To Write Chemical Equations From Word Descriptions YouTube
https://i.ytimg.com/vi/npyvZSBqyc0/maxresdefault.jpg
Writing Formula Equations Ws 1 - This chemical formula writing worksheet for set 1 comes in a variety of formats making it adaptable to your classroom and their needs Included alongside the worksheet is also a sheet devised for SEND students and all answers are included Chemical formula writing has been developed to make sure that equations and formula can be