Write The Balanced Equation For The Reaction Shown Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products In chemical reactions atoms are never created or destroyed
The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter Co A 3 a q Ni s Co A 2 a q Ni A 2 a q Is this equation balanced It appears to be balanced with respect to mass since there is one Co atom and one Ni atom on each side of the equation
Write The Balanced Equation For The Reaction Shown

Write The Balanced Equation For The Reaction Shown
https://www.wikihow.com/images/6/63/Balance-Chemical-Equations-Step-7Bullet3-Version-2.jpg
SOLVED Write The Balanced Chemical Equation For The Reaction Shown
https://cdn.numerade.com/ask_images/0cadebafbdd5418cad2dc8415dad5533.jpg
Chemical Writing Balanced Equations Upper Sec Science
http://joannewong.weebly.com/uploads/2/5/9/3/25936499/_247105566.jpg
Phase 2 Chemical Problem Solving N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have
Instructions on balancing chemical equations Enter an equation of a chemical reaction and click Balance The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character Examples Fe Au Co Br C O N F Compare Co cobalt and CO carbon monoxide A redox equation can be balanced using the following stepwise procedure 1 Divide the equation into two half reactions 2 Balance each half reaction for mass and charge 3 Equalize the number of electrons transferred in each half reaction 4 Add the half reactions together In this video we ll use these steps to balance the redox
More picture related to Write The Balanced Equation For The Reaction Shown
Solved Write The Balanced Chemical Equation For The Reaction Chegg
https://media.cheggcdn.com/media/d9c/d9c136bd-1a07-4ea4-8b70-21ab50b0383e/phpYUPpBG.png
Answered Write The Balanced Chemical Equation Bartleby
https://prod-qna-question-images.s3.amazonaws.com/qna-images/question/3b108657-cfac-4b11-a602-2fad68a5ad17/b912f827-e3bb-45f7-a387-b06014e22d53/88fk5hm_processed.jpeg
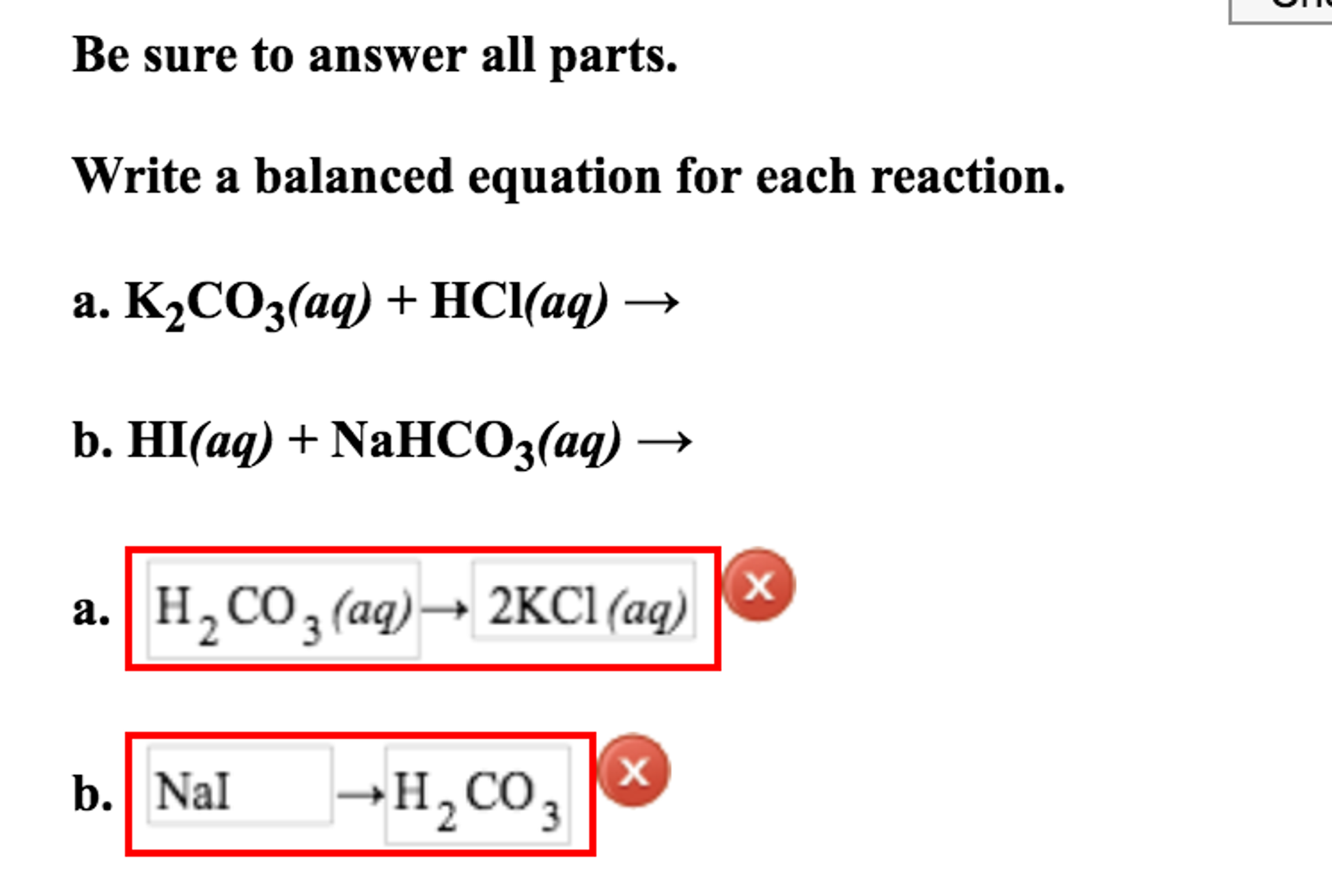
Solved Write A Balanced Equation For Each Reaction Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/8c2/8c2e8a8b-309d-4370-ad4e-59961327b0d1/phpHTYgO5.png
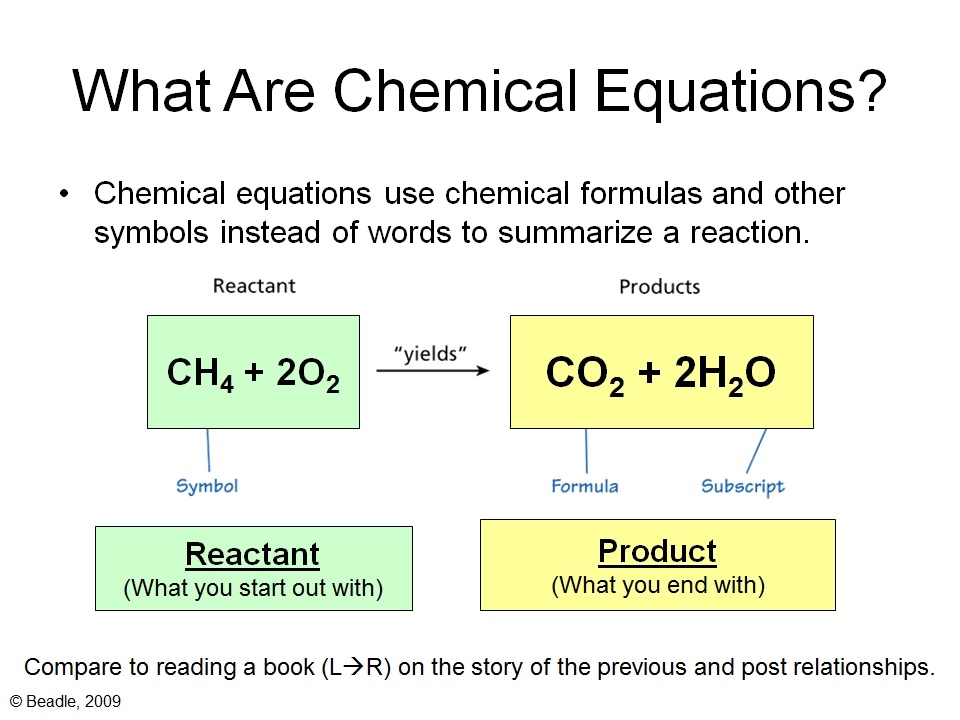
3 Steps for Balancing Chemical Equations 1 Write the unbalanced equation Chemical formulas of reactants are listed on the lefthand side of the equation Products are listed on the righthand side of the equation Reactants and products are separated by putting an arrow between them to show the direction of the reaction Balancing Equations A balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
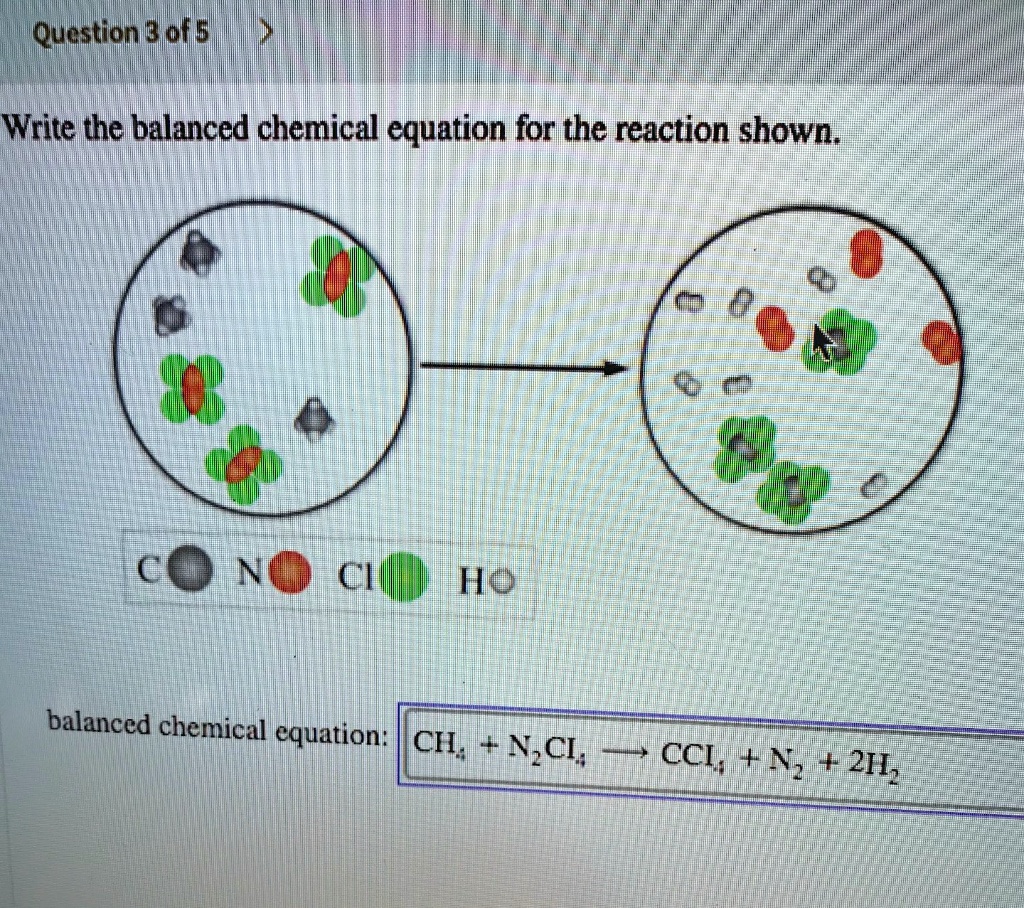
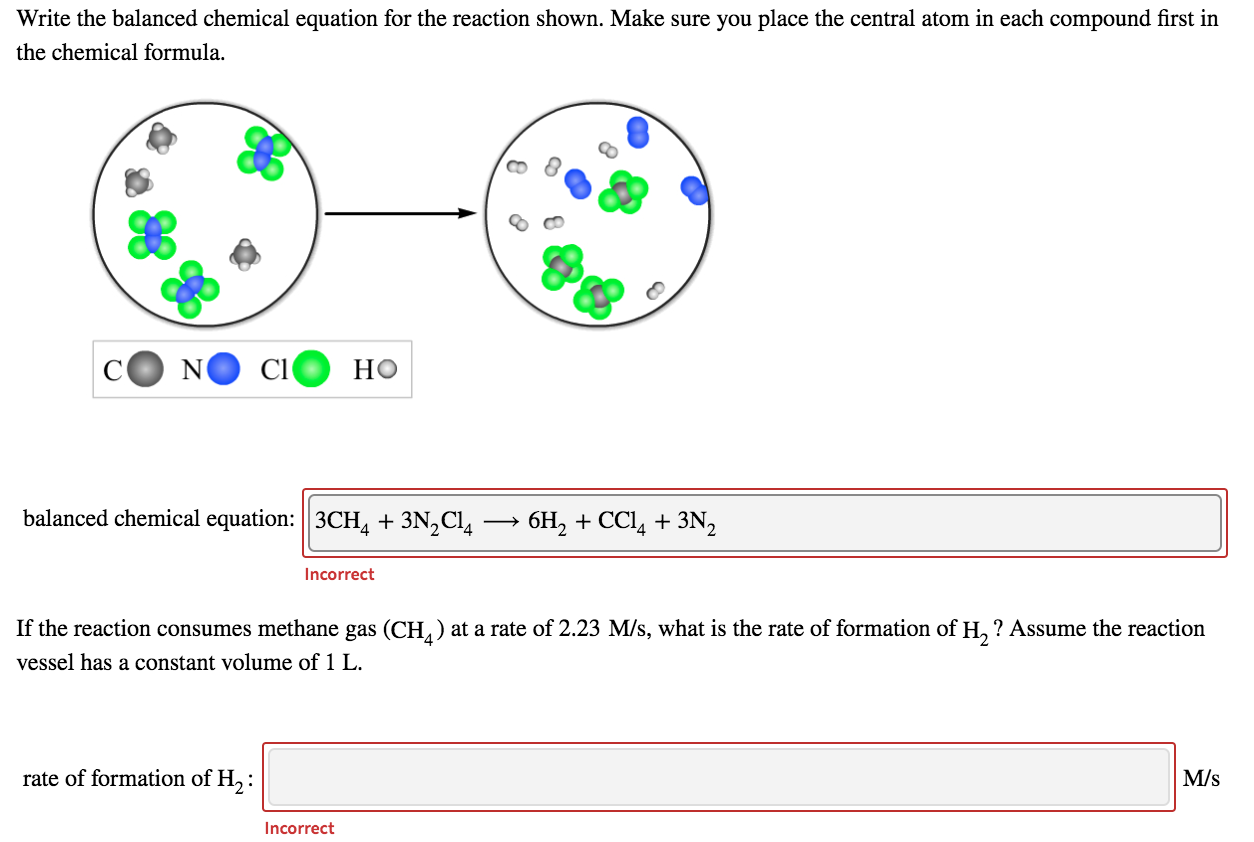
Question 1a Textbook Question The reaction of A2 red spheres with B2 blue spheres is shown in the diagram What is the balanced chemical equa tion LO 3 1 a 2 A2 6 B2 4 AB3 b 4 A 12 B 4 AB3 c 4 A 12 B A4 B12 d A2 3 B2 2 AB3 293 1 Question 1b Textbook Question Make sure you place the central atom in each compound first in the chemical formula N CI balanced chemical equation If the reaction consumes methane gas CH at a rate of 1 10 M s what is the rate of formation of H

How To Balance Chemical Equations 10 Steps with Pictures
http://www.wikihow.com/images/b/b8/Balance-Chemical-Equations-Step-10Bullet1-Version-2.jpg
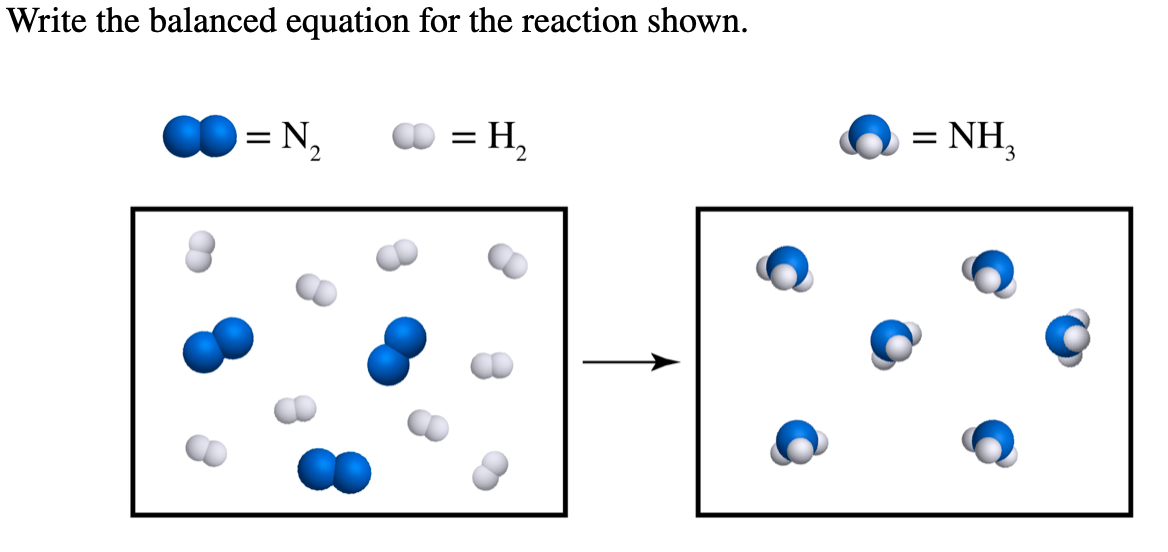
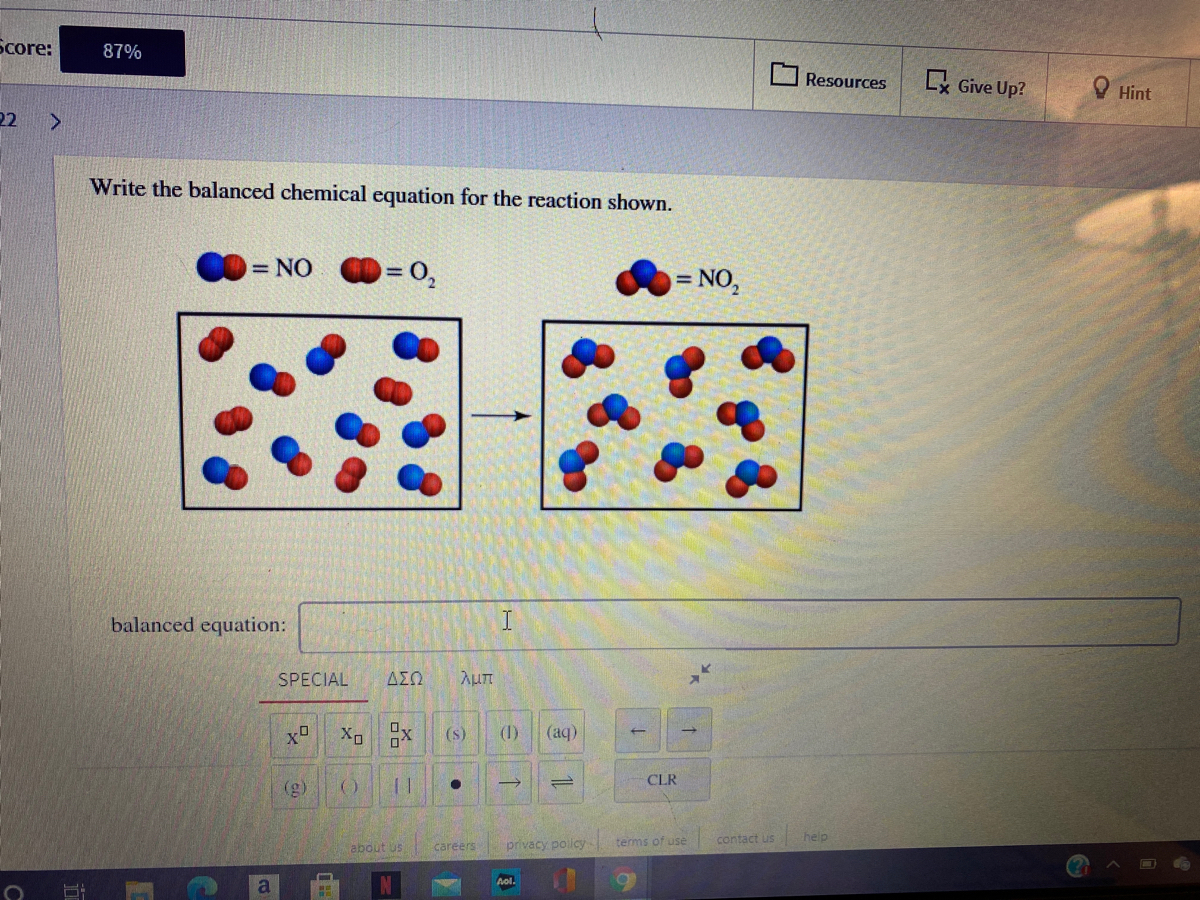
Solved Write The Balanced Equation For The Reaction Shown Chegg
https://media.cheggcdn.com/media/588/588d48cf-f63b-4599-b9f3-9856cbbbb25a/phpBEk4UM.png
Write The Balanced Equation For The Reaction Shown - Instructions on balancing chemical equations Enter an equation of a chemical reaction and click Balance The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character Examples Fe Au Co Br C O N F Compare Co cobalt and CO carbon monoxide