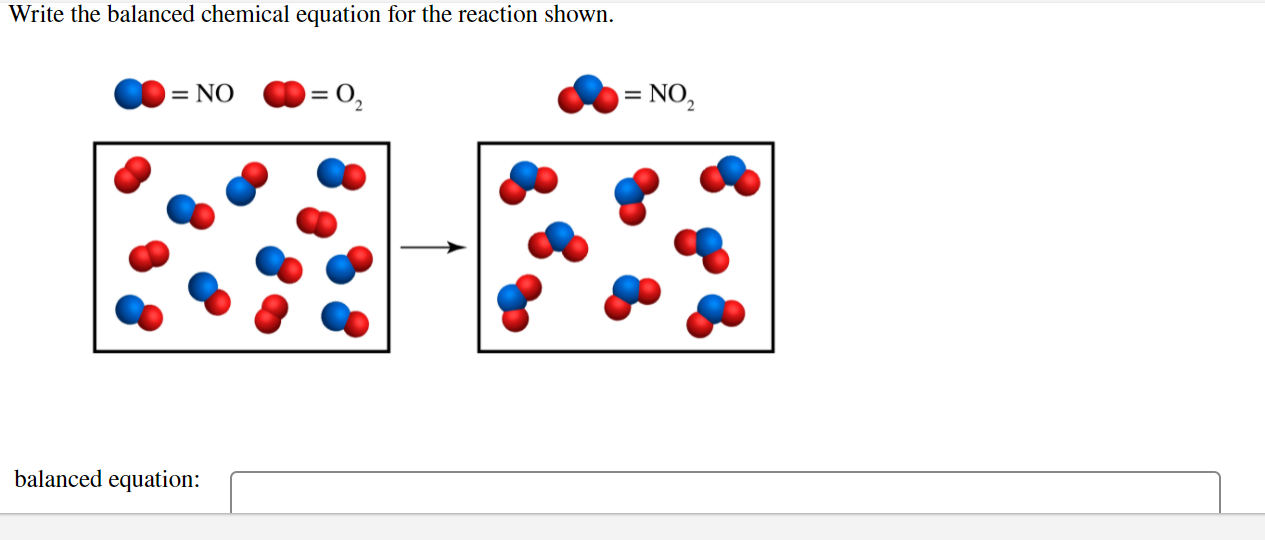
Write The Balanced Chemical Equation For The Reaction Shown Balancing Equations The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
5 2 10 2 5 10 10 10 yes The numbers of N and O atoms on either side of the equation are now equal and so the equation is balanced Exercise 4 1 1 4 1 1 Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen molecular oxygen and water A balanced chemical equation shows the same number of each type of atom on both sides of the arrow Questions Tips Thanks Want to join the conversation Sort by Top Voted Gabrielle M 9 years ago I m working on Chemical Reactions Double and Single Replacement on FLVS
Write The Balanced Chemical Equation For The Reaction Shown

Write The Balanced Chemical Equation For The Reaction Shown
https://www.wikihow.com/images/6/63/Balance-Chemical-Equations-Step-7Bullet3-Version-2.jpg
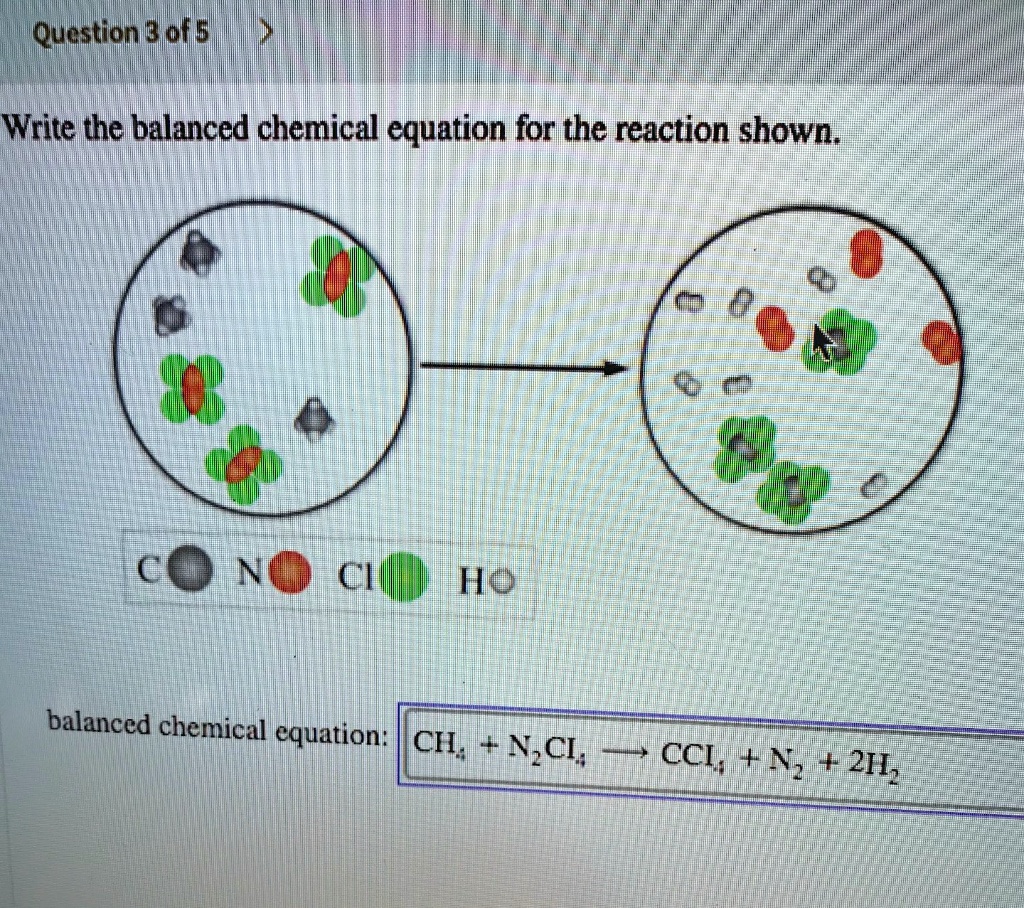
SOLVED Write The Balanced Chemical Equation For The Reaction Shown
https://cdn.numerade.com/ask_images/0cadebafbdd5418cad2dc8415dad5533.jpg

Write The Balanced Chemical Equation For The Reaction Shown Brainly
https://us-static.z-dn.net/files/d24/e8e2d1d5dfc10482fcf05ea34e874aba.png
Question 1a Textbook Question The reaction of A2 red spheres with B2 blue spheres is shown in the diagram What is the balanced chemical equa tion LO 3 1 a 2 A2 6 B2 4 AB3 b 4 A 12 B 4 AB3 c 4 A 12 B A4 B12 d A2 3 B2 2 AB3 293 1 Question 1b Textbook Question Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the equation and only
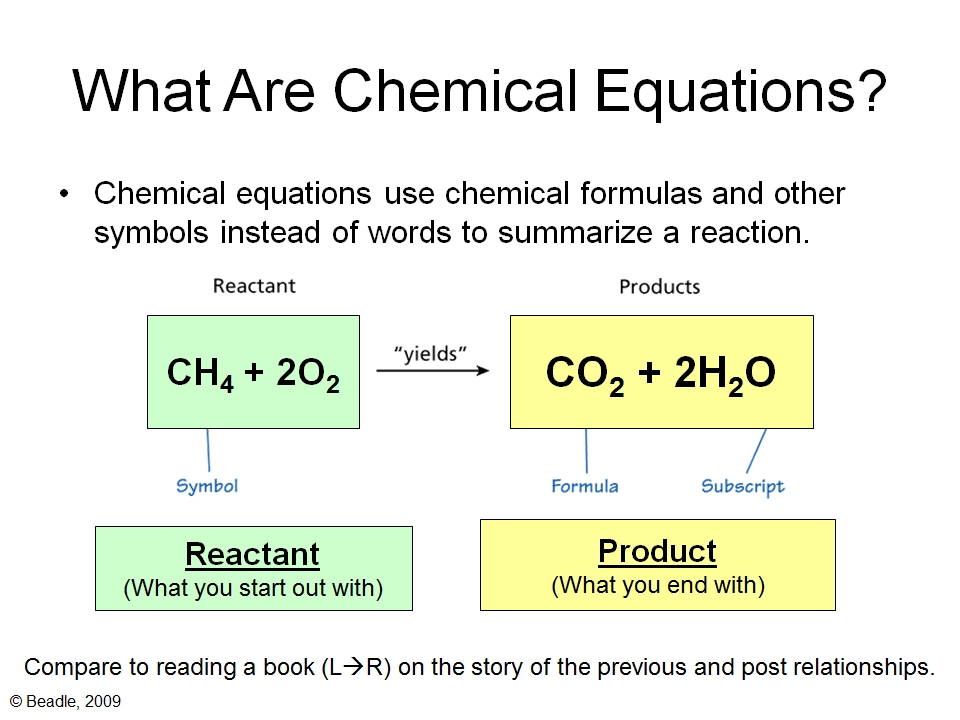
3 Steps for Balancing Chemical Equations 1 Write the unbalanced equation Chemical formulas of reactants are listed on the lefthand side of the equation Products are listed on the righthand side of the equation Reactants and products are separated by putting an arrow between them to show the direction of the reaction A balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
More picture related to Write The Balanced Chemical Equation For The Reaction Shown
Solved Write The Balanced Chemical Equation For The Reaction Chegg
https://media.cheggcdn.com/media/6b9/6b9583a6-7b7c-48e9-b1d9-e9be867e981c/phpRsRzlB
Balancing Chemical Equations VISTA HEIGHTS 8TH GRADE SCIENCE
https://vhmsscience8.weebly.com/uploads/1/2/7/6/12762866/445853_2_orig.jpg
How To Balance Chemical Equations solutions Examples Videos
http://www.onlinemathlearning.com/image-files/xbalance-chemical-equations.png.pagespeed.ic.mtPzrx5_1V.png
To balance a redox equation using the half reaction method the equation is first divided into two half reactions one representing oxidation and one representing reduction The equations for the half reactions are then balanced for mass and charge and if necessary adjusted so that the number of electrons transferred in each equation is the same The requirement of charge balance is just a specific type of mass balance in which the species in question are electrons An equation must represent equal numbers of electrons on the reactant and product sides and so both atoms and charges must be balanced Step 5 Balance charge by adding electrons
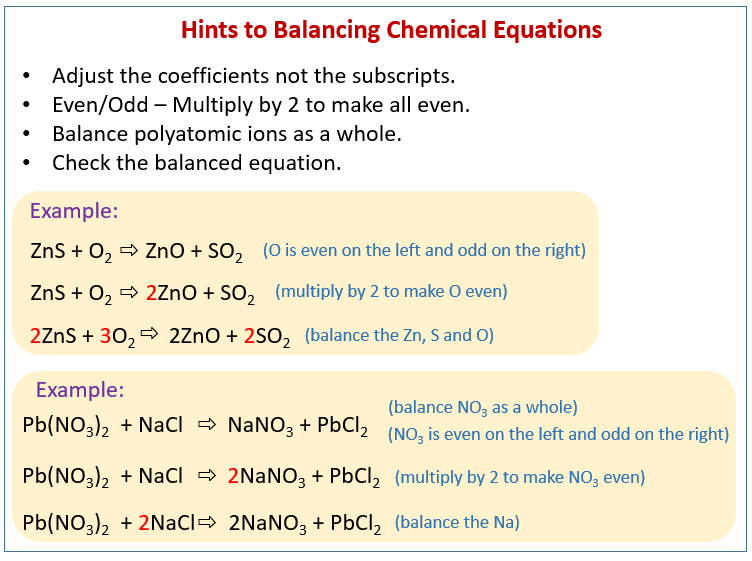
2 2 4 1 2 2 1 4 4 4 yes A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection Consider as an example the decomposition of water to yield molecular hydrogen and oxygen This process is represented Easy Steps for Balancing Chemical Equations Follow four easy steps to balance a chemical equation Write the unbalanced equation to show the reactants and products Write down how many atoms of each element there are on each side of the reaction arrow Add coefficients the numbers in front of the formulas so the number of atoms of each
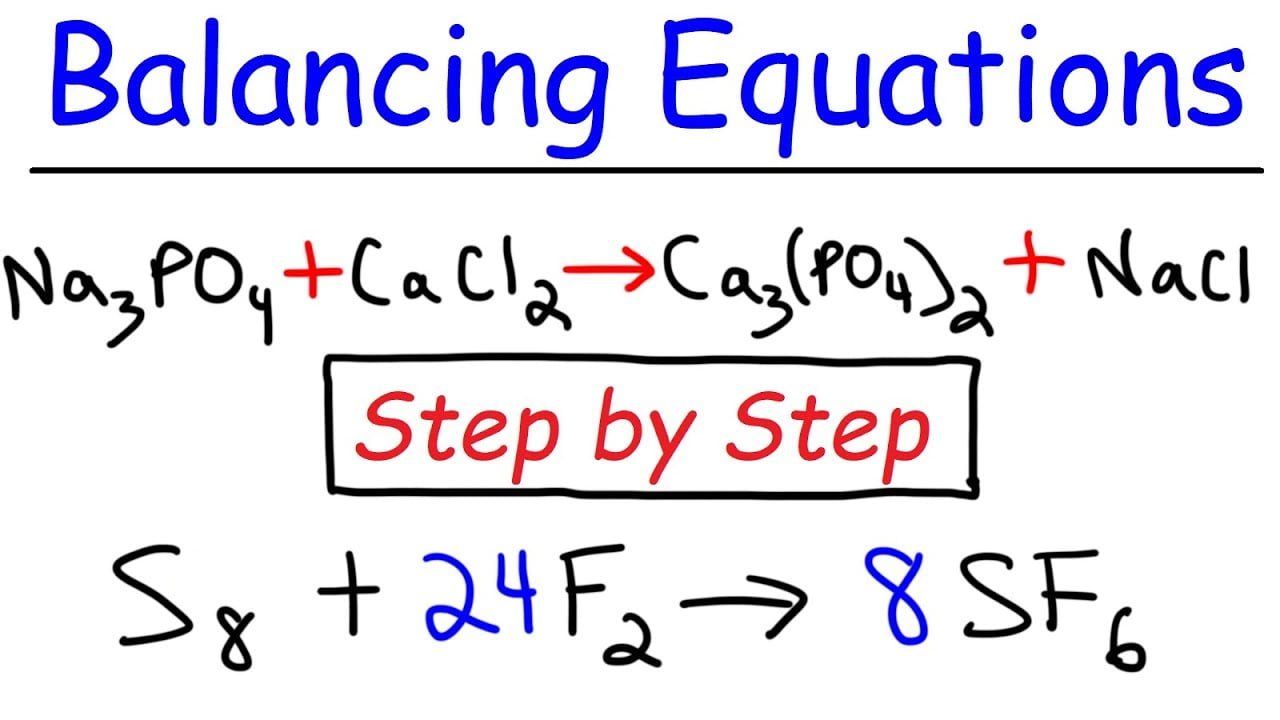
Easy Way Of Balancing Chemical Equations And Exercise Solutions
https://nkedugists.com.ng/wp-content/uploads/2020/05/Balancing-of-chemical-equation.jpg

How To Balance Chemical Equations 10 Steps with Pictures
http://www.wikihow.com/images/9/98/Balance-Chemical-Equations-Step-10Bullet1.jpg
Write The Balanced Chemical Equation For The Reaction Shown - These coefficients yield equal numbers of both H and O atoms on the reactant and product sides and the balanced equation is therefore 2H 2O 2H 2 O 2 Example 1 7 1 Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen N 2 and oxygen O 2 to form dinitrogen pentoxide