Worksheet Reaction Rates Answers What is the average rate of the reaction for the first minute Second minute Overall Time min Concentration M 0 5 0M 1 3 0M 2 2 0M a Using the graph below calculate the rate of the reaction between the second and the fifth minute Rate slope 44mL 10mL 11 3 mL min When is the rate of the reaction the greatest
Q4 Experimentally Determining the Rate Law Determine the rate law and value of k k for th e following reaction at 800 oC in a closed vessel Remember how concentration is related to pressure within the Ideal Gas law 2H2 g 2NO g 2H2O g N2 g 2 H 2 g 2 NO g 2 H 2 O g N 2 g Exp Worksheet 14 Chemical Kinetics Given the following energy diagram for a 3 step reaction answer the questions below Use your graph to determine the instantaneous reaction rate at 250 ms Given that the reaction is first order in NO and in O 3 determine the rate constant using your calculated rate for each set of data points
Worksheet Reaction Rates Answers
Worksheet Reaction Rates Answers
https://rocketsheets.co.uk/wp-content/uploads/rates-of-reaction-home-learning-worksheet-gcse-12462015-preview-3.png

Chemical Reactions Worksheet Answers
https://s3.paperzz.com/store/data/008694134_1-09883b7d29ff66a51cd042bd262d3936.png

Rate Of Reaction Practical Home Learning Worksheet GCSE Rocketsheets
https://rocketsheets.co.uk/wp-content/uploads/rate-of-reaction-practical-home-learning-worksheet-gcse-12462009-preview-1.png
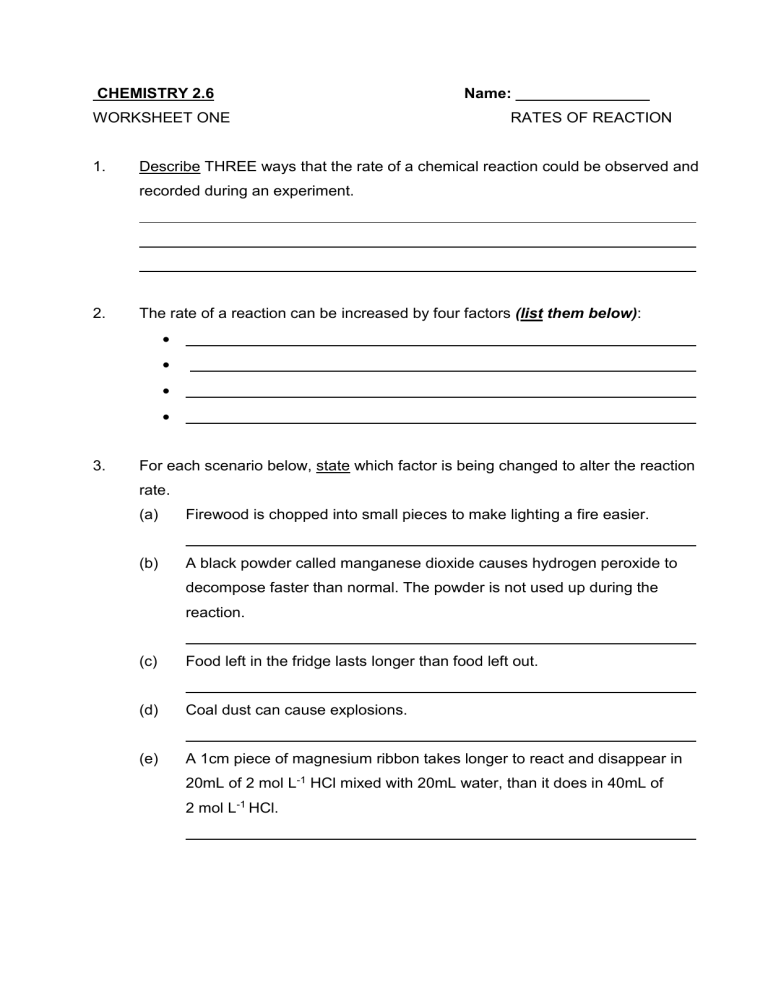
1 A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid The equation for the reaction is Zn s 2HCl aq o H2 g ZnCl2 aq A piece of zinc is dropped into 1 00 L of 0 100 M HCl and the following data were obtained Time Mass of Zinc 0 s 4 s 8 s 12 s 16 s 20 s 0 016 g Microsoft Word ws8s09key doc CH302 Worksheet 15 on Kinetics Answer Key 1 What factors determine the rate of a reaction How does each of them affect the rate Answer Rate xAe Ea RT Shown in this equation the factors affecting rate of reaction are concentration and order of reactants and products x which increases as rate
Want to play around with the reactions and rate PHET Simulation Click on the image below and download The rate of the reaction is determined by measuring the volume of H A 2 g produced every five seconds Which of the following experimental changes is most likely to decrease the rate of H A 2 g production Choose 1 answer Using a 0 080 g sample of powdered Mg s instead of Mg s ribbon A
More picture related to Worksheet Reaction Rates Answers
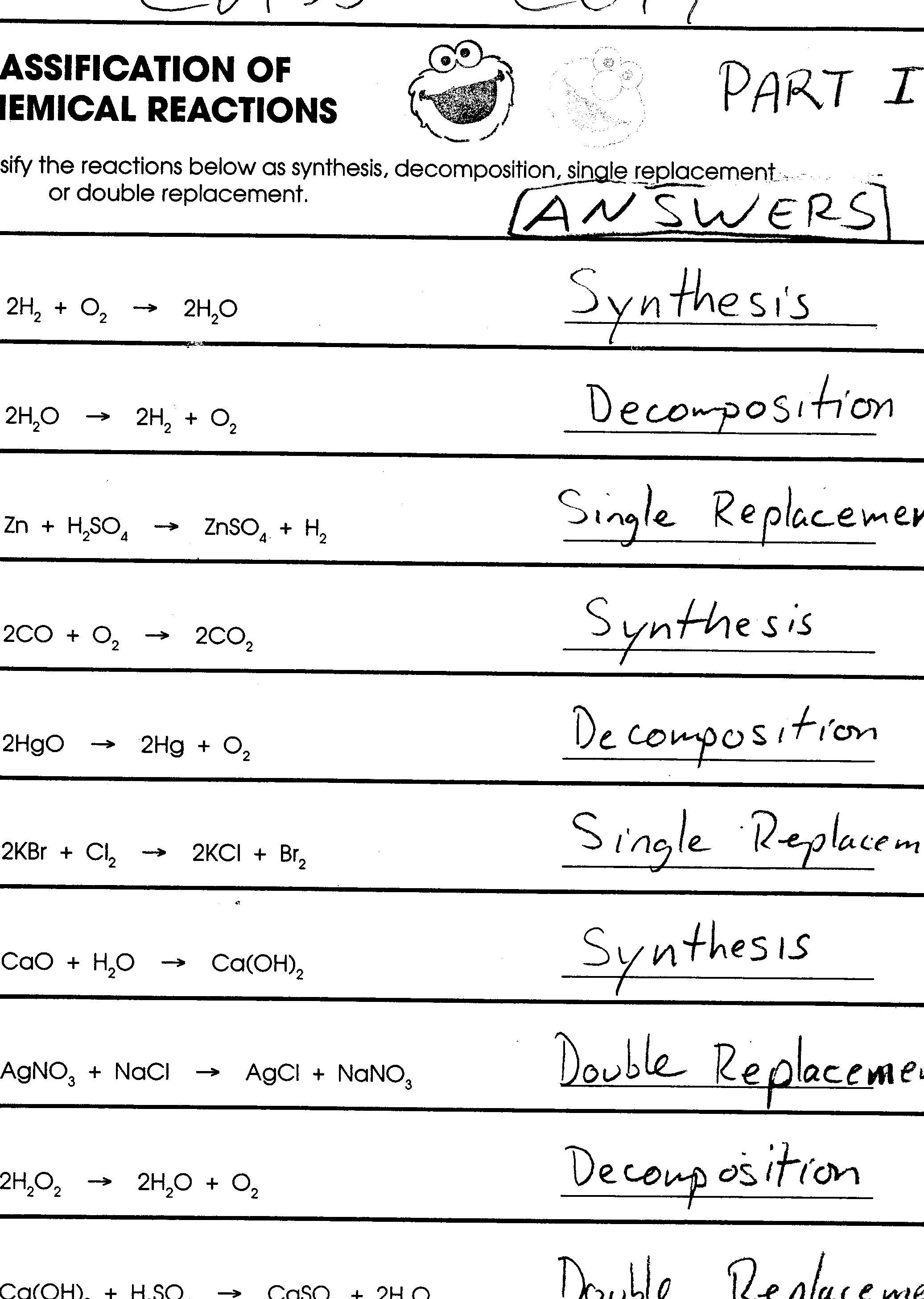
10 Types Of Reactions Worksheet Worksheeto
https://www.worksheeto.com/postpic/2011/07/classifying-chemical-reactions-worksheet-answer-key_278921.jpg

13 Best Images Of Worksheet Reaction Rates Answer Worksheet Measuring
http://www.worksheeto.com/postpic/2012/04/chemical-reaction-types-worksheet_240189.jpg
3 Worksheet Rates Of Reactions
https://s3.studylib.net/store/data/025428385_1-1001ac6f2d167fb7198aea6f9418757a-768x994.png
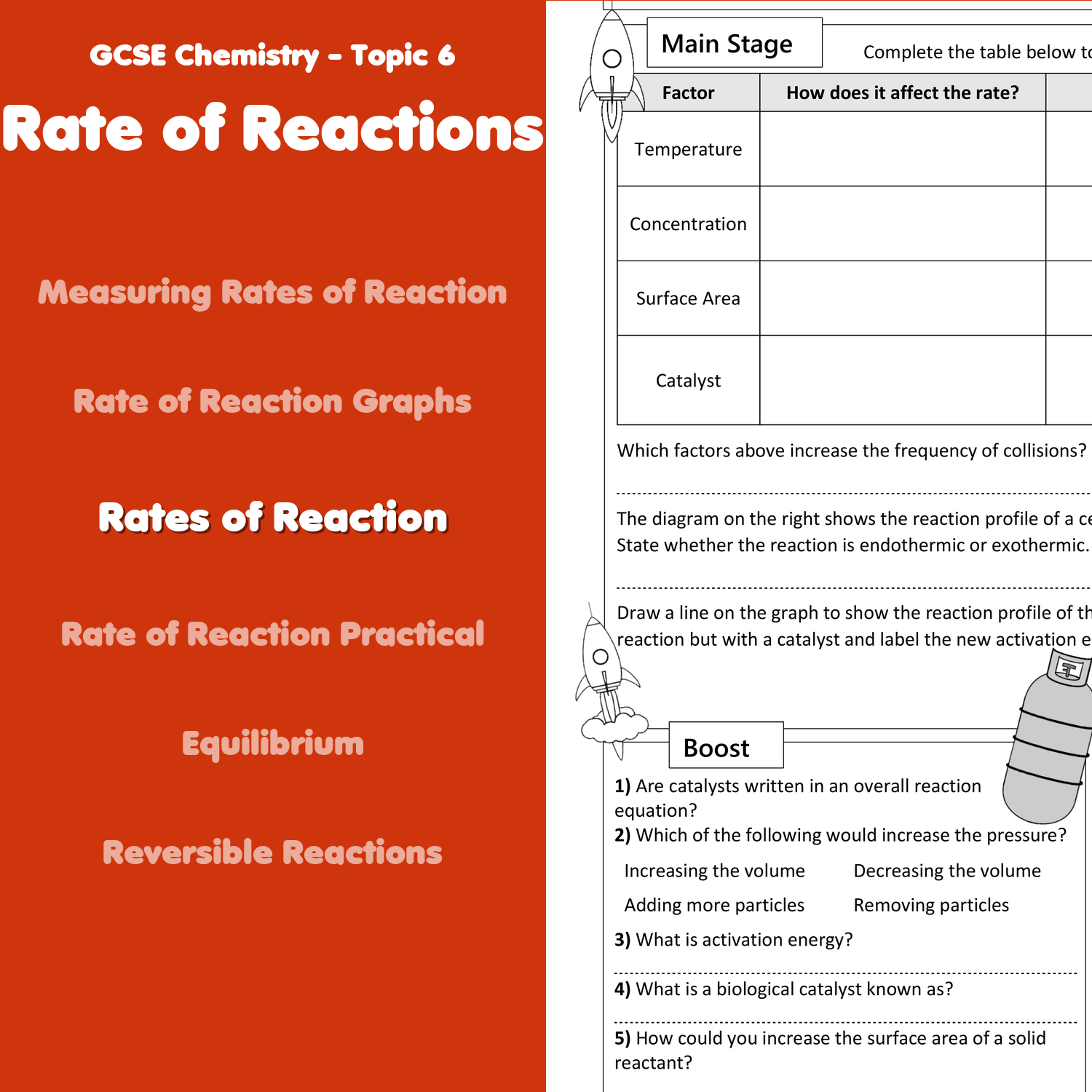
To summarise the rate of reaction can be affected by 1 temperature 2 particle size and 3 concentration of the reactants Still another factor which can change a reaction s rate is a catalyst This is a substance which changes the rate of a reaction but remains itself un changed 1 What happens to the concentrations of a C6H12O6 O2 as the reaction proceeds b H2O CO2 as the reaction proceeds 2 According to the collision theory what 3 circumstances are needed for C6H12O6 O2 to react 3 What is the activation energy for a chemical reaction 4 Use the equation the collision theory to explain
9 Enzymes are in molds and bacteria that spoil food Explain using your knowledge of factors affecting the rate of reaction why food doesn t spoil as fast when it is refrigerated as it would at room temperature 10 Due to decomposition reactions with oxygen or carbon dioxide in the air meat begins to feel slimy and smell spoiled 1 A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid The equation for the reaction is Zn s 2HO aq f H2 g ZnC12 aq A piece of zinc is dropped into 1 00 L of 0 100 MHCI and the following data were obtained T une f j Mass 0 Zmc Os 4 S 8 S 12 s 16 s 20 s 0 016 g 0 014 g 0 012 g
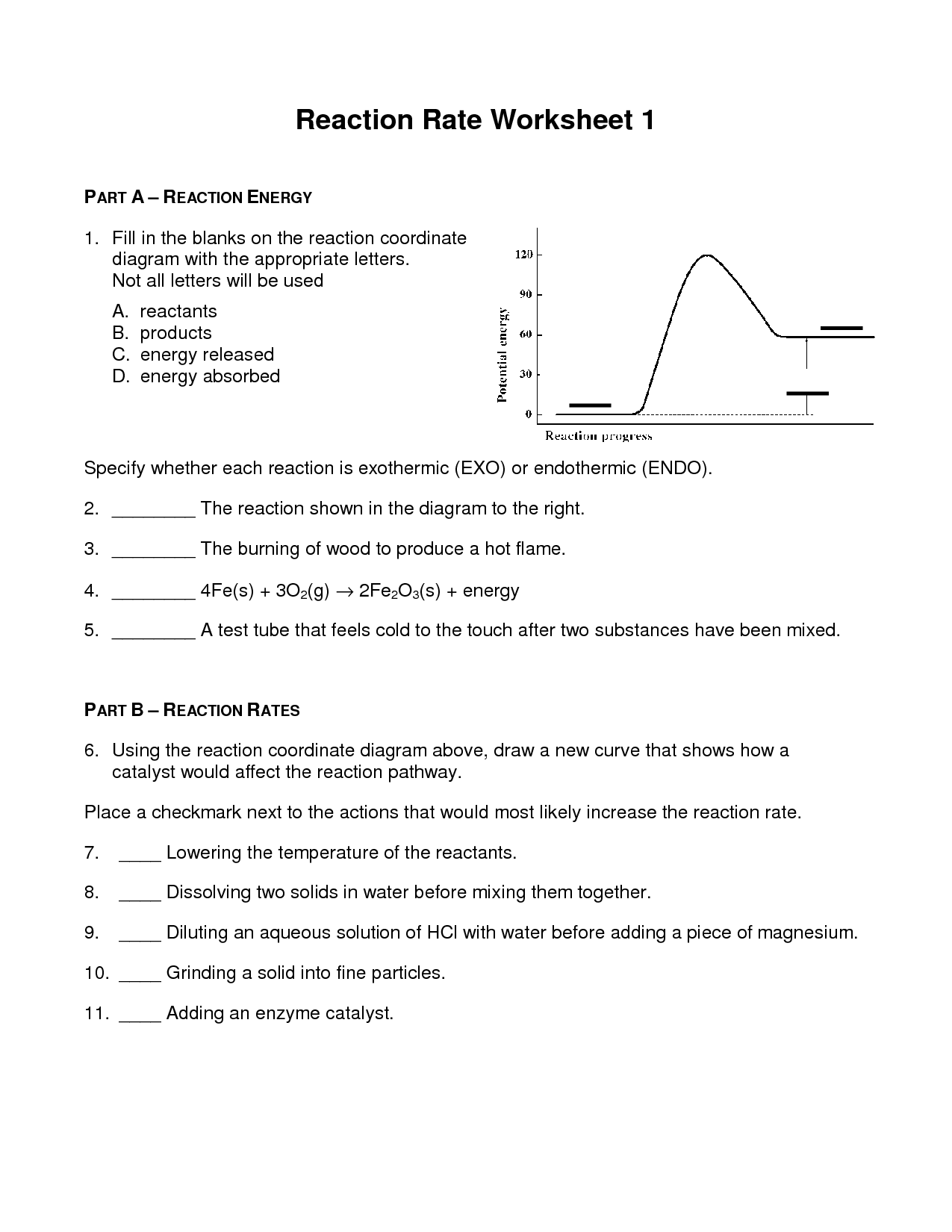
13 Worksheet Reaction Rates Answer Worksheeto
https://www.worksheeto.com/postpic/2012/04/exothermic-reaction-worksheet_240182.png
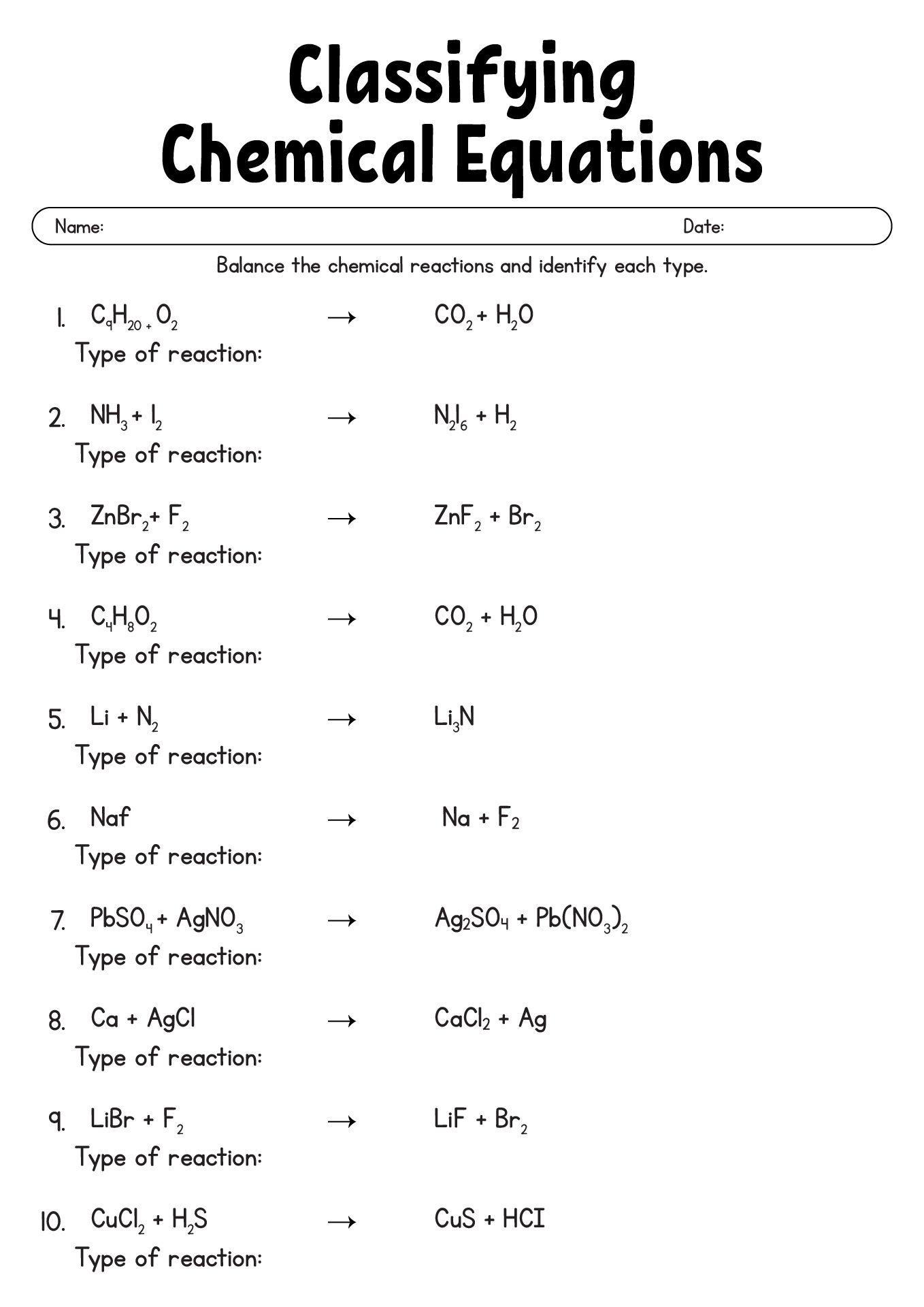
17 Chemical Reactions Worksheet With Answers Worksheeto
https://www.worksheeto.com/postpic/2011/07/chemical-reaction-types-worksheet_236693.png
Worksheet Reaction Rates Answers - Rate of reaction No Answer 10 The reaction of hydrochloric acid with magnesium is a redox reaction 2HCl aq Mg s MgCl2 aq H2 g The higher concentration of hydrochloric acid ensures that more molecules of HCl are available to react with the magnesium metal and so the rate at which the reaction proceeds is greater Page 2 Pearson