Worksheet 4 Single Replacement Reactions ACTIVITY SERIES REFERENCE CHART In general a metal can displace any of the metals which are lower in the reactivity series Same rule applies for Halogens Metals from Li to Na will replace H from acid and water Metals from Mg to Pb will replace H from acids only ANSWER KEY Ag KNO3 NR Zn 2AgNO3 2Ag Zn NO3 2
A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound Single Replacement Reaction Worksheet For each reaction use the activity series to complete the reaction If no reaction will occur write no reaction Make sure you balance your final answer Ag KNO3 Zn AgNO3 Cl2 KI Cu FeSO4 Fe Pb NO3 2 Cu Al2 SO4 3 Al Pb NO3 2 Cl2 NaI Fe AgC2H3O2 Al CuCl2
Worksheet 4 Single Replacement Reactions

Worksheet 4 Single Replacement Reactions
https://i0.wp.com/www.neshaminy.org/cms/lib/PA01000466/Centricity/Domain/205/Single Replacemment.jpg

Worksheet 4 Single Replacement Reactions 50 Classifying Chemical
https://cdn.slidesharecdn.com/ss_thumbnails/lab5-labsample-120222123004-phpapp01-thumbnail-4.jpg?cb=1329913913

Single Replacement Reaction Worksheets
https://www.housview.com/wp-content/uploads/2018/11/double_replacement_reaction_worksheet_5_5.jpg
A single replacement reaction is a reaction in which one element replaces a similar element in a compound The general form of a single replacement also called single displacement reaction is A BC AC B A BC AC B In this general reaction element A A is a metal and replaces element B B also a metal in the compound A single replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products Presented below 2HCl aq Zn s ZnCl2 aq H2 g 2 HCl aq Zn s ZnCl 2 aq H 2 g is an example of a single replacement reaction
REACTION If the reaction occurs WRITE THE CORRECT FORMULAS for the reactants and products Remember the following elements are diatomic H2 N2 O2 F2 Cl2 Br2 I2 Use the chart of polyatomic ions and transition metals if necessary BALANCE the equation 1 and 5 are done already as examples Ni CuCl2 Cu NiCl2 Predict the products and balance the following single replacement reactions If no reaction occurs write N R For transition metals use the following charges Iron Fe3 Mercury Hg2 Lead Pb4 Copper Cu1 Gold Au3 Fe CuCl2 Hg Sn SO4 2 Ba Ni3 PO4 2 Pb Au NO3 3 Li HOH K AgCl Ca NaOH
More picture related to Worksheet 4 Single Replacement Reactions

Single Replacement Reactions Worksheet Answer Key Jands stage cl tutorial
https://static.docsity.com/documents_first_pages/2021/04/20/49d5f42f7d8af62c2bf2ece17a080768.png
Types Of Chemical Reactions Practice Worksheet
http://media.cheggcdn.com/media/3c6/3c6a6191-1439-4c90-906b-0dede3113e2f/image

Chemistry Replacement Reaction Worksheet
https://s3.studylib.net/store/data/008707860_1-df3eea4a6f73f9467eb38fba77e85801.png
Pound This replacement is usually limited to the halogens F2 Cl2 Br2 and I2 The activity of the halogens decreases as you go down the Group 17 of the periodic table 1 AB C CB A 2 A BC BA C In these reactions a free element reacts with a compound Single and Double Replacement Reactions Worksheet Created by All Your Chemistry Needs After going over slides 8 10 from the Unit 7 PowerPoint the student can practice writing and balancing single and double replacement reactions in this worksheet Subjects Chemistry Grades 10 th 12 th Types Worksheets Activities Handouts
Question Worksheet 4 4 Predicting Single Replacement Reactions Predict the products and balance the following single replacement reactions If no reaction occurs write N R For transition metals use the following charges 1 Fe CuCl aq 2 Hgm Sn SO4 2 aq 3 Ba Ni3 PO4 2 aq 4 Pb Au NO3 3 29 5 Lio HOH 1 6 Predicting and Balancing Single Replacement Reactions Single replacement reactions involve reacting a lone element usually a metal with a compound resulting in the cations switching places Students will learn to use the activity series for the first time as part of this worksheet as they identify when reactions will and will not occur

Single Replacement Reaction Worksheets Answers Key
https://i.pinimg.com/originals/1e/6d/c6/1e6dc60bcc138bad961e9f4bc94b48a4.jpg
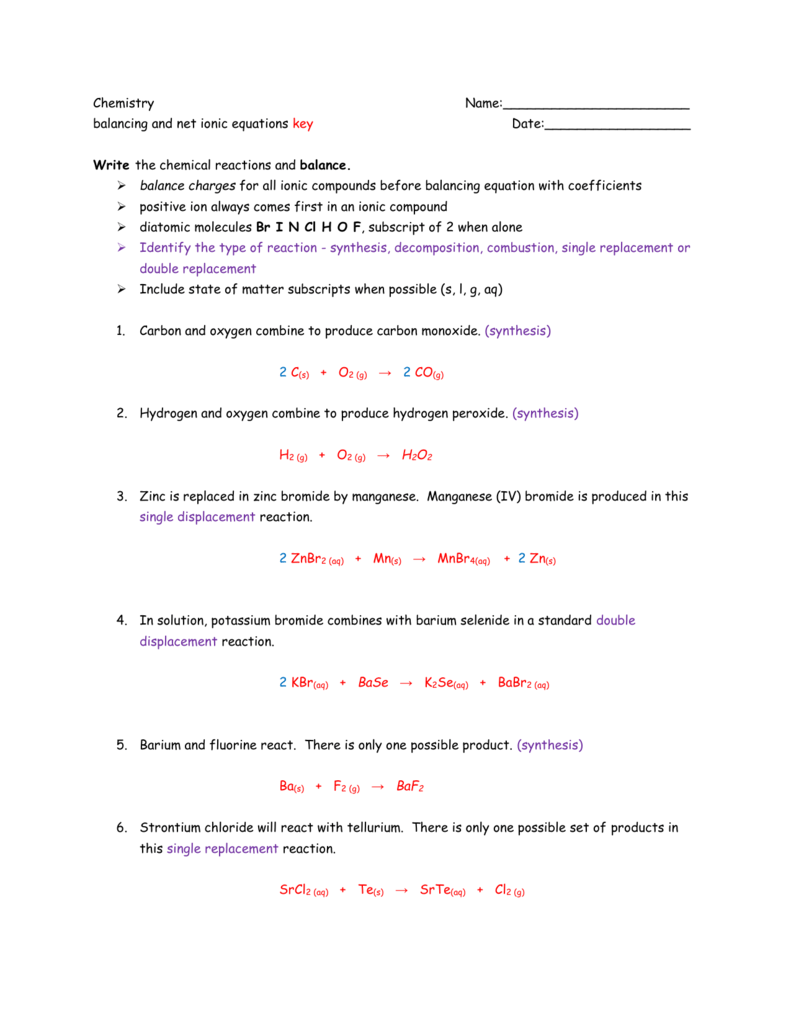
Single Replacement Reactions Worksheets Answer Key
https://s3.studylib.net/store/data/007130368_1-56af4f7ae60ae152e3d0dd193eedf922.png
Worksheet 4 Single Replacement Reactions - A single replacement reaction is a reaction in which one element replaces a similar element in a compound The general form of a single replacement also called single displacement reaction is A BC AC B A BC AC B In this general reaction element A A is a metal and replaces element B B also a metal in the compound
