When The Following Equation Is Balanced The Coefficients Are 1 When the following equation is balanced the coefficients are C8H18 O2 CO2 H2O A 2 3 4 4 B 1 4 8 9 C 2 12 8 9 D 4 4 32 36 E 2 25 16 18 Click the card to flip E Click the card to flip 1 53 Flashcards Learn Test Match Q Chat Created by Muhlenberg Students also viewed CHEM Chapter 3 Bimodal Questions
Balance a chemical equation when given the unbalanced equation Explain the role of the Law of Conservation of Mass in a chemical reaction Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the equation and only
When The Following Equation Is Balanced The Coefficients Are

When The Following Equation Is Balanced The Coefficients Are
https://www.wikihow.com/images/6/63/Balance-Chemical-Equations-Step-7Bullet3-Version-2.jpg
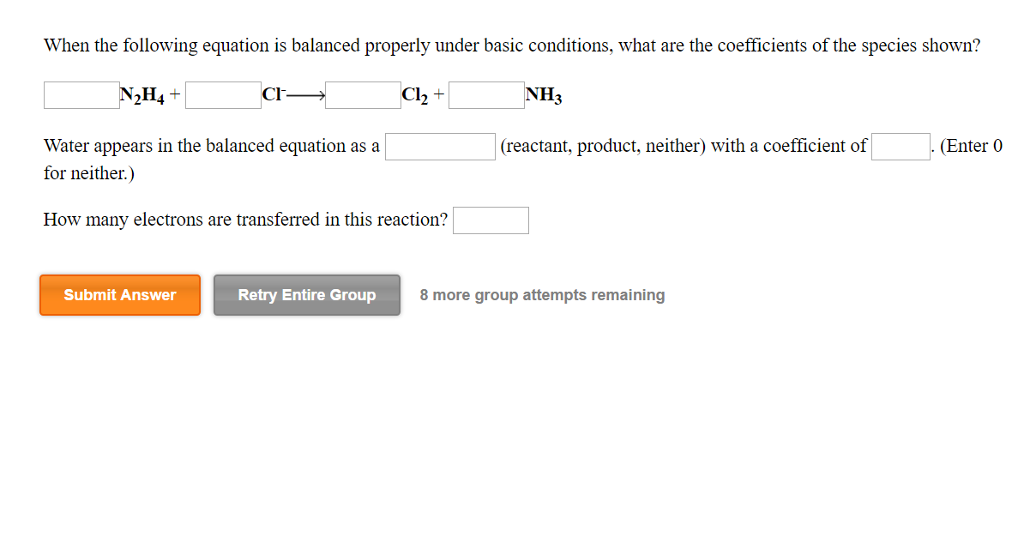
Solved When The Following Equation Is Balanced Properly Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/5bd/5bd516d9-b438-4ca3-88c6-7cbb353800bd/phpzQL8xv.png

Balance The Following Chemical Equation By Providing The Correct
https://us-static.z-dn.net/files/d68/fec66a0b572c586ed6661628071aaf21.jpeg
To balance a redox equation using the half reaction method the equation is first divided into two half reactions one representing oxidation and one representing reduction The equations for the half reactions are then balanced for mass and charge and if necessary adjusted so that the number of electrons transferred in each equation is the same The balanced equation must now be used to convert moles of Fe s to moles of H 2 g Remember that the balanced equation s coefficients state the stoichiometric factor or mole ratio of reactants and products 3 74 x 10 5 mol Fe s 1mol H 2 g 1mol Fe s 3 74 x 10 5 mol H 2 g Step 5 Check units
N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have An equation is balanced by changing coefficients in a somewhat trial and error fashion It is important to note that only the coefficients can be changed NEVER a subscript the coefficient times the subscript gives the total number of atoms Four examples before balancing the equation d 3Ca NO Now back to balancing the example equation
More picture related to When The Following Equation Is Balanced The Coefficients Are

How To Change Coefficient To Balance Chemical Equations YouTube
https://i.ytimg.com/vi/zab7tgSXxuE/maxresdefault.jpg
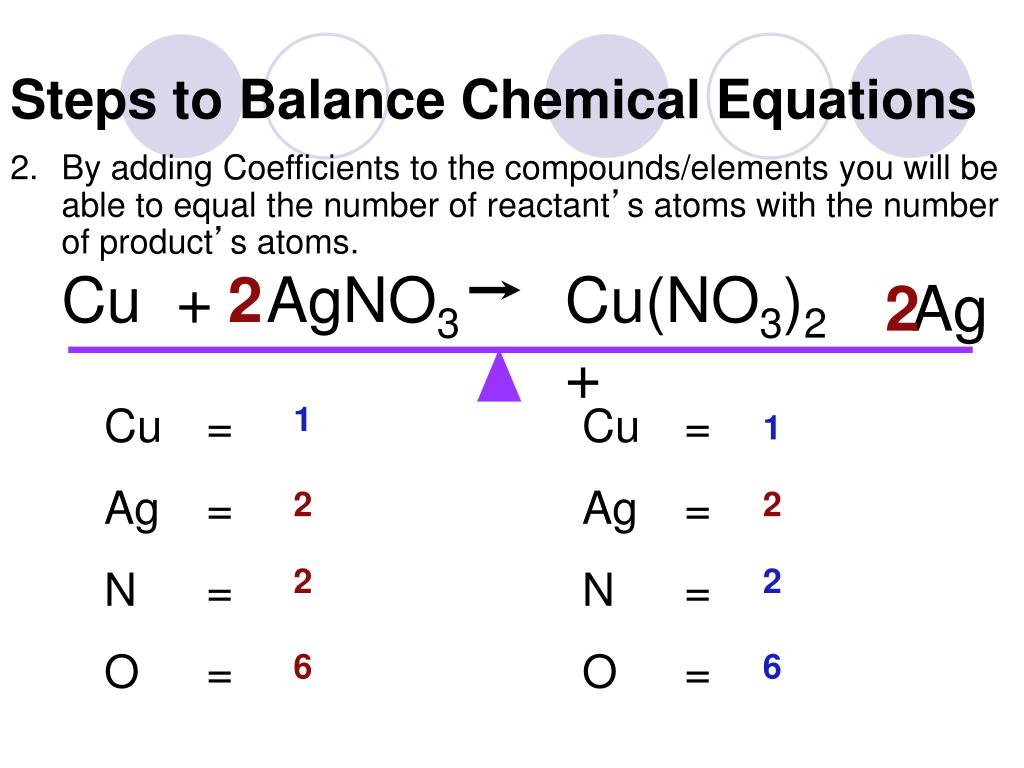
PPT How To Balance Chemical Equations PowerPoint Presentation Free
https://image2.slideserve.com/3850828/steps-to-balance-chemical-equations1-l.jpg
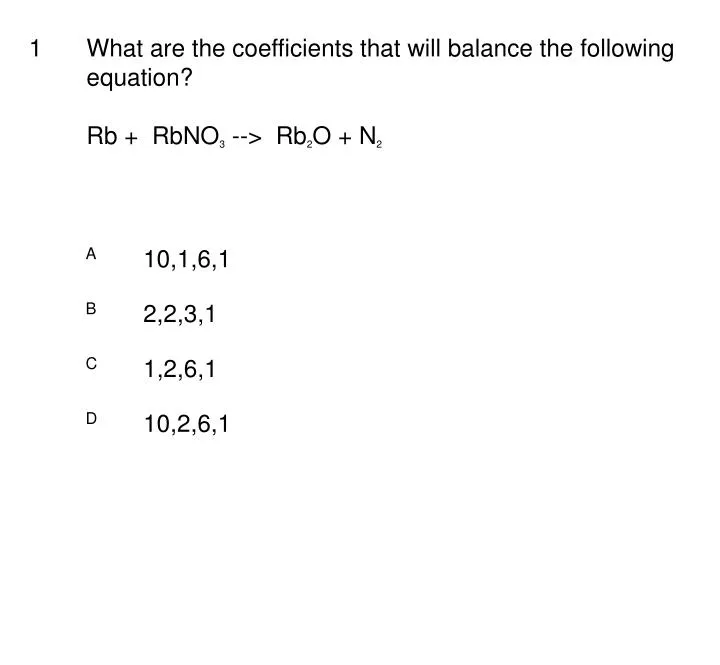
PPT What Are The Coefficients That Will Balance The Following
https://image2.slideserve.com/3938738/slide1-n.jpg
Key Questions What law is satisfied by a balanced chemical equation in chemistry Conservation of matter is the law You can also call it the conservation of mass When we balance an equation we determine the ratio of reactants to products which allows for the total number of atoms of reactants to match the number of atoms of the products To balance a chemical equation enter an equation of a chemical reaction and press the Balance button The balanced equation will appear above Use uppercase for the first character in the element and lowercase for the second character Examples Fe Au Co Br C O N F Ionic charges are not yet supported and will be ignored
When the following equation is balanced the coefficients are C8H18 O2 CO2 H2O Click the card to flip 2 25 16 18 Click the card to flip 1 20 Flashcards Learn Test Match Q Chat Consuelo Landeros Top creator on Quizlet Students also viewed CHEM Chapter 3 Bimodal Questions 69 terms Cindy8744 Preview CHEM 1341 EXAM 2 82 terms When the coefficients in a balanced equation are multiplied by a common factor the equilibrium constant is raised to the power of the corresponding factor As before pure solids and liquids are not accounted for in the equation K c and K p are related by the following equation K p K c RT Delta n nonumber where Delta n

When The Following Molecular Equation Is Physical Chemistry
https://media.kunduz.com/media/question/seo/raw/20210321024549500182-3040657_7Wz2QWxDN.jpeg?h=512
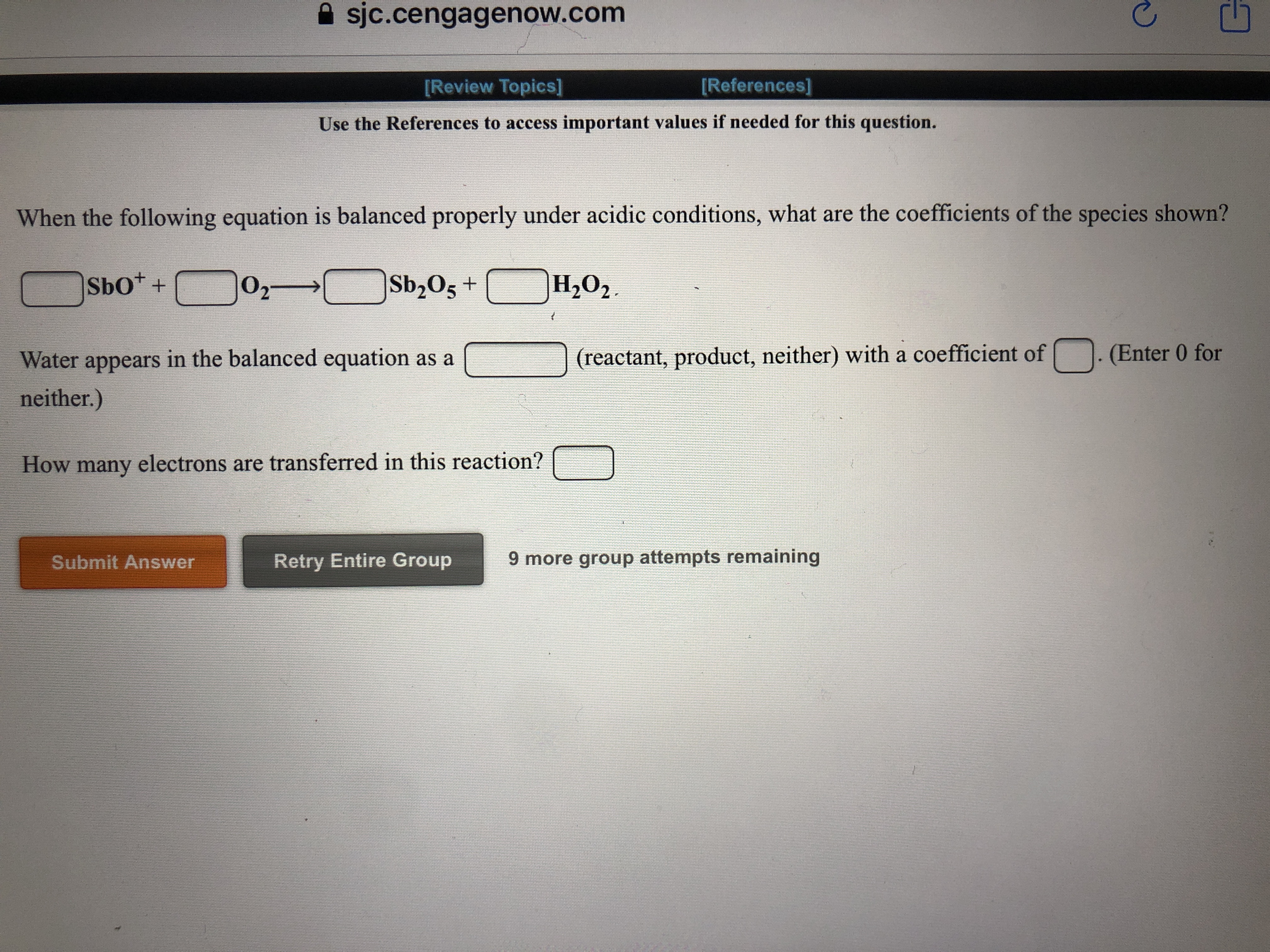
Answered When The Following Equation Is Balanced Bartleby
https://prod-qna-question-images.s3.amazonaws.com/qna-images/question/8a698ba8-107e-4192-8f35-9ba49cc09e89/9ab62c3d-817e-4965-9a7b-812dd2a49b02/dxpc10m.jpeg
When The Following Equation Is Balanced The Coefficients Are - Instructions on balancing chemical equations Enter an equation of a chemical reaction and click Balance The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character Examples Fe Au Co Br C O N F Compare Co cobalt and CO carbon monoxide