Unit Stoichiometry Mole Mole Calculations Ws 1 1 How many moles of potassium oxide K O will be formed when 1 52 moles of potassium reacts with oxygen according to the following reaction 4K O2 2 K20 2 How many moles of copper are needed to react with sulfur to produce 0 25 moles of copper t sulfide according to the following reaction 2 Cu 5 Cu 7 01 3
In this article we ll look at how we can use the stoichiometric relationships contained in balanced chemical equations to determine amounts of substances consumed and produced in chemical reactions Balanced equations and mole ratios This stoichiometry video tutorial explains how to perform mole to mole conversions from a balanced chemical equation It contains plenty of examples of mole
Unit Stoichiometry Mole Mole Calculations Ws 1

Unit Stoichiometry Mole Mole Calculations Ws 1
http://www.worksheeto.com/postpic/2013/10/mole-calculation-worksheet-answer-key_708267.jpg
Stoichiometry Mole Unit Stoichiometry
https://imgv2-2-f.scribdassets.com/img/document/320185115/original/1c240dc309/1563712188?v=1
Compound Stoichiometry Mole Conversions 2019 YouTube
https://i.ytimg.com/vi/IVD6Kmdtrfo/maxresdefault.jpg
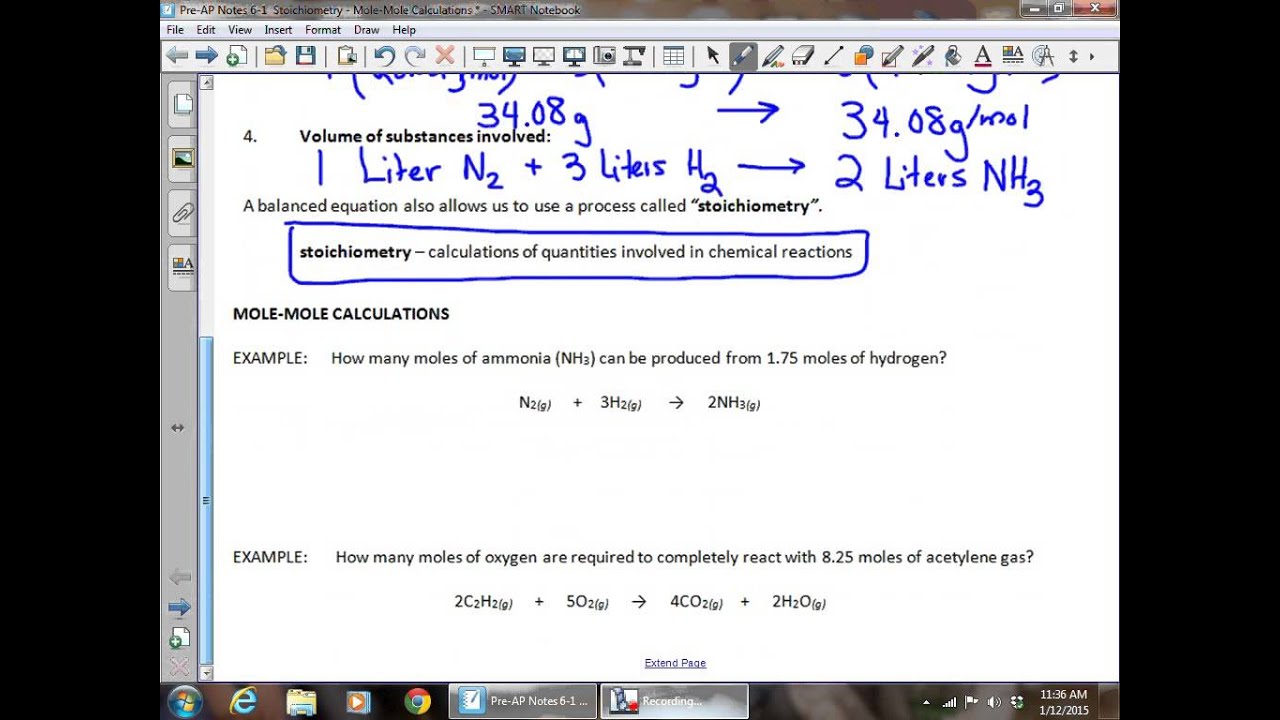
1 The chemical equation of interest is this O CuSO 2 Every one mole of CuSO 4 5H 2 O that is heated releases five moles of water The ratio from the chemical equation is this 3 The ratio from the problem data is this 4 Solving x 8 75 moles of water will be produced Stoichiometry Worksheet 1 Mole to Mole Calculations Learning Target Students will calculate the theoretical yield in moles You must soive each of the followins problems using dimensional analysis EVERY ucnber tb 1 For this reaction AI your work should be fblowed by a unit nd formula a
Introduction to General Chemistry Malik 4 Stoichiometry the quantification of chemical reactions Practice Mole calculations Stoichiometry No videos or articles available in this lesson Quiz 2 Unit test Get ready to better understand chemical reactions with stoichiometry Master the art of measuring substances using Avogadro s number and explore how the mighty mole helps us predict the outcomes of chemical reactions
More picture related to Unit Stoichiometry Mole Mole Calculations Ws 1

Mole Mole Stoichiometry Worksheet Worksheets For Kindergarten
https://s2.studylib.net/store/data/009852559_1-2ca2e812702e78f5bccb5e1b51a4f290-768x994.png
Pre AP Video Notes 6 1 Stoichiometry Mole Mole Calculations YouTube
https://i.ytimg.com/vi/QkZBQWn2TlU/maxresdefault.jpg
9 Best Images Of Chemistry Conversion Worksheets With Answers Mass To
http://www.worksheeto.com/postpic/2012/03/mass-to-mole-stoichiometry-worksheet-answer-key_471420.jpg
Unit test Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class education for anyone anywhere Stoichiometry Worksheet 1 Mole to Mole Calculations Learning Target Students will calculate the theoretical yield in moles Directions You must solve each of the following problems using dimensional analysis EVERY number in your work should be followed by a unit and a formula 1 For this reaction Al O Al 2O 3 a
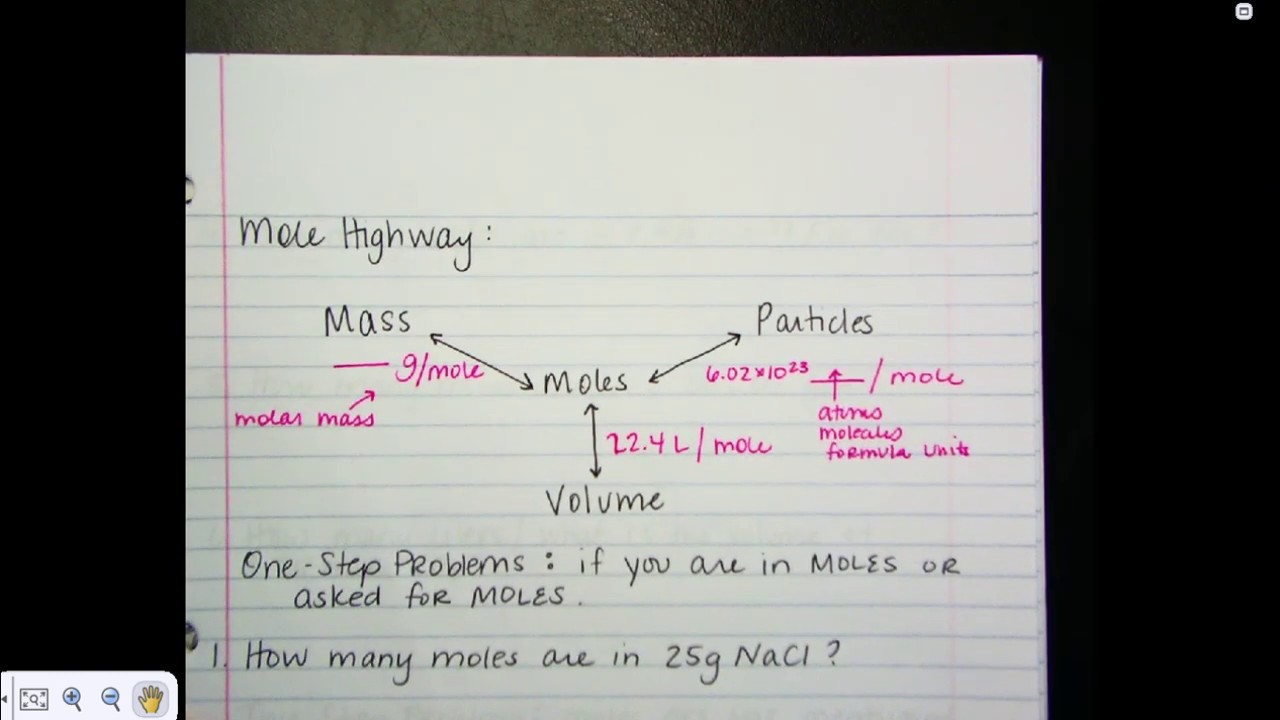
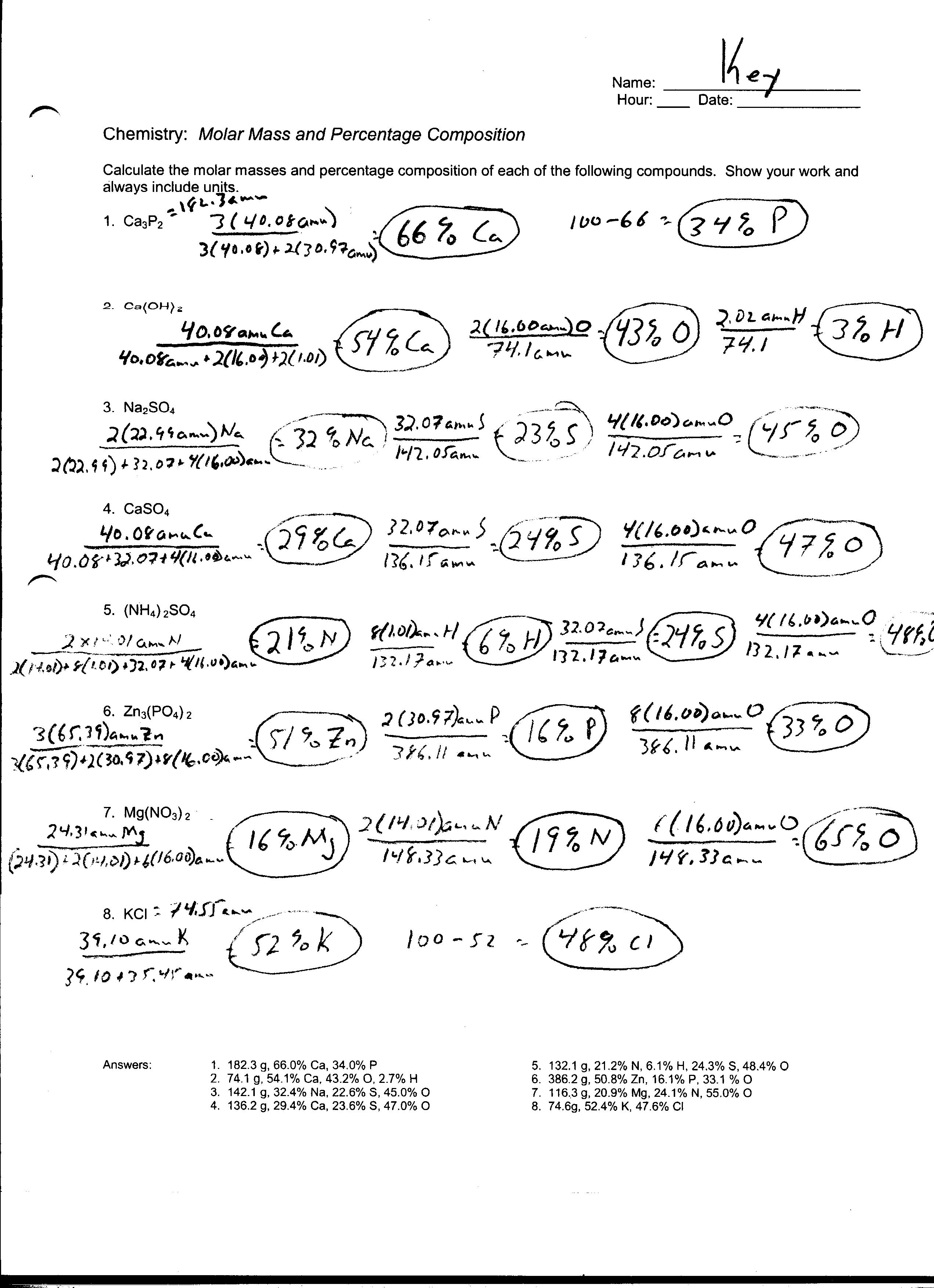
Given the balanced equation 2 N2H4 l N2O4 l 3 N2 g 4 H2O g A How many moles of dinitrogen tetrahydride are required to produce 57 moles of nitrogen B How many moles of dinitrogen tetroxide are required to produce 57 moles of nitrogen C How many moles of water are produced when 57 moles of nitrogen are made 3 The formula mass of a substance is the sum of the atomic masses of its atoms The molar mass gram formula mass of a substance equals one mole of that substance 3 3e The empirical formula of a compound is the simplest whole number ratio of atoms of the elements in a compound

Grade 10 Chemistry Stoichiometry Test moles
https://s3.studylib.net/store/data/008937319_1-7b61860744ce3df6c23146b5b52b729a.png

Labster Stoichiometric Calculations Answers Quizlet CALCULATORJHS
https://i2.wp.com/www.worksheeto.com/postpic/2010/10/stoichiometry-practice-worksheet-answer-key_224021.jpg
Unit Stoichiometry Mole Mole Calculations Ws 1 - Mole Mole Calculations Using Mole Ratios Using Ratios in Mathematical Problems If I told you that you a Chicago Hot Dog costs 2 you could draw ratios from that statement 1 Chicago Hot Dog 2 or more importantly you could write the ratio as conversion factors A fairly easy question to ask you would be If you had 10 how may hot dogs
