Unit Stoichiometry Mole Mole Calculations Ws 1 Answers BU Chem 103 Christianson Phase 2 Chemical Problem Solving
1 The chemical equation of interest is this O CuSO 2 Every one mole of CuSO 4 5H 2 O that is heated releases five moles of water The ratio from the chemical equation is this 3 The ratio from the problem data is this 4 Solving x 8 75 moles of water will be produced Step 1 Convert known reactant mass to moles In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio we first need to know the quantity of H A 2 SO A 4 in moles We can convert the 3 10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 98 08 g mol
Unit Stoichiometry Mole Mole Calculations Ws 1 Answers
Unit Stoichiometry Mole Mole Calculations Ws 1 Answers
https://imgv2-2-f.scribdassets.com/img/document/261361842/original/d41b05e83c/1567532458?v=1

Mole Mole Stoichiometry Worksheet Worksheets For Kindergarten
https://s2.studylib.net/store/data/009852559_1-2ca2e812702e78f5bccb5e1b51a4f290-768x994.png

Chemistry Mole Calculation Worksheet Worksheet
https://files.liveworksheets.com/def_files/2020/2/18/2181717497365/2181706596362002.jpg
CONCEPT 1 BASIC MOLE CONCEPT CALCULATIONS Number of mole number of particles Avogadro s constant i e 6 1023 Number of mole mass g mol 1 molar mass g Number of mole volume of gas molar volume of gas i e 24 dm3 at r t p Number of mole concentration volume of solution Y3EOY DHS 2008 I 20 Avogadro s Number One of the most important ideas in chemistry is the mole concept A mole of substance is that amount that contains as many elementary units atoms molecules or formula units depending on the nature of the substance as there are atoms in exactly 12 grams of an isotopically pure sample of 12 C 12 g 12 C exactly mole 12 C atoms
In all cases quantities of a substance must be converted to moles before the balanced chemical equation can be used to convert to moles of another substance 5 6 Yields 5 7 Limiting Reagents The limiting reagent is the reactant that produces the least amount of product Mass mass calculations can determine how much product is produced and Unit test Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class education for anyone anywhere
More picture related to Unit Stoichiometry Mole Mole Calculations Ws 1 Answers
AP Chemistry Stoichiometry Practice Problems With Answers Mole
https://imgv2-2-f.scribdassets.com/img/document/246736266/original/1c4a5d8899/1587965430?v=1
Stoichiometry Practice 2 Worksheet Answers Printable Word Searches
https://i2.wp.com/lh3.googleusercontent.com/proxy/YLfQO2Ktop3x54BhOh9tk13b7HDzDXRDMDkZ58yQ1ElhX3qHqEpxKSglUvtaUfhfmNx-mpIWj4NApaAlHN8bvPRDERlmJSpAcXXzhst3hzZG-KDFUVYdCrSlipnNYpAJ=w1200-h630-p-k-no-nu

Molar Mass Practice Worksheet Answers
http://www.worksheeto.com/postpic/2013/10/mole-calculation-worksheet-answer-key_708267.jpg
High school chemistry 9 units 56 skills Unit 1 Atoms elements and the periodic table Unit 2 Chemical bonding Unit 3 Chemical reactions Unit 4 Stoichiometry and the mole Unit 5 States of matter Unit 6 Thermochemistry Unit 7 Solutions acids and bases Unit 8 Reaction rates and equilibrium VIDEO ANSWER In this video we want to discuss a mole to mole stoichiometry operation So in order to do stoichiometry you need first a balanced equation Unit Stoichiometry Mole Mole Calculation WS 1 Video Answer Solved by verified expert Instant Answer Step 1 4 1 Write a balanced chemical equation for the reaction Step 2 4 2
The formula mass of a substance is the sum of the atomic masses of its atoms The molar mass gram formula mass of a substance equals one mole of that substance 3 3e The empirical formula of a compound is the simplest whole number ratio of atoms of the elements in a compound 7 Worksheets in Moles Stoichiometry Practice converting moles Chemical reactions give information about the amount of MOLES involved the reaction The coefficients are the relative amounts of moles of each reactant and product used or produced in the reaction A mole ratio relates the proportions of moles of any 2
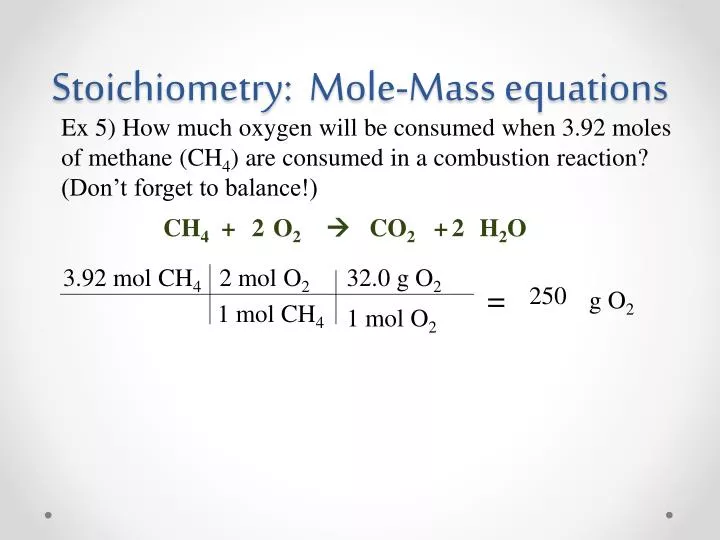
PPT Stoichiometry Mole Mass Equations PowerPoint Presentation Free
https://image2.slideserve.com/4011225/stoichiometry-mole-mass-equations-n.jpg

Teach Your Students To Complete Mole to liter mole to volume
https://i.pinimg.com/originals/e7/99/0b/e7990b8ad502dabd90a5477f6445bb9e.png
Unit Stoichiometry Mole Mole Calculations Ws 1 Answers - Advertisement Chemistry 11 Unit 7 Stoichiometry Worksheet 7 1 Stoichiometric Calculations Mole to Mole Calculations 1 The octane present in gasoline burns according to the following equation 2C8H18 25O2 16CO2 18H2O a How many moles of O2 are needed to react fully with 7 moles of octane C8H18 87 5 mol O2 b How many moles of CO2 can

