Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers Example 1 How many grams of hydrogen gas are needed to react completely with 54 0 g of oxygen gas given the following unbalanced chemical reaction 1 Balance the chemical equation 2 Convert grams of the substance given 54 0 g 32 0 g mol 1 6875 mol of O Note the use of 32 0 and not 16 0
Solution We will simply follow the steps mass K mol K mol Mg mass Mg In addition to the balanced chemical equation we need the molar masses of K 39 09 g mol and Mg 24 31 g mol In one line 86 4 gK 1molK 39 09 gK 1 molMg 2 molK 24 31gMg 1 molMg 26 87gMg Exercise 5 5 3 We can use that ability to answer stoichiometry questions in terms of the masses of a particular substance in addition to moles We do this using the following sequence Collectively these conversions are called mole mass calculations As an example consider the balanced chemical equation The mol Fe 2 O 3 units cancel leaving mol SO 3 unit
Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers

Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers
https://media.madebyteachers.com/wp-content/uploads/2023/01/25053852/stoichiometry-mass-to-mass-10-prev.png

Stoichiometry Mass To Mass Worksheet Answers
https://i.ytimg.com/vi/anh4rGF_3QU/maxresdefault.jpg

Mole Mole Stoichiometry Worksheet Answers
https://s3.studylib.net/store/data/007734847_2-ccaf4ade60b8a5a6d3eb03b9a1dd62e3.png
How do you find mass using molar mass If the moles of a substance are known the mass can be determined by multiplying the number of moles by the molar mass of the substance What Is PRACTICE 1 KCl Br2 KBr Cl2 How many moles of chlorine are produced from 0 37 moles of potassium chloride PRACTICE 2 C3H8 O2 CO2 H2O How many moles of oxygen are needed to produce 2 04 moles of carbon dioxide
Step 1 Convert known reactant mass to moles In order to relate the amounts H A 2 SO A 4 and NaOH using a mole ratio we first need to know the quantity of H A 2 SO A 4 in moles We can convert the 3 10 grams of H A 2 SO A 4 to moles using the molar mass of H A 2 SO A 4 98 08 g mol Mole mass calculation where we start with a given number of moles of a substance and calculate the mass of another substance involved in the chemical equation or vice versa For example suppose we have the balanced chemical equation 2Al 3Cl 2 2AlCl 3 Suppose we know we have 123 2 g of Cl 2
More picture related to Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers

12 Best Images Of Mole Ratio Worksheet Answer Key Mole Ratio
http://www.worksheeto.com/postpic/2015/07/mole-ratio-worksheet-answers_224142.png

Unit 2 Molar Mass Worksheet
https://i.pinimg.com/736x/eb/a3/d7/eba3d7451df51dffbb3eb18cac1869d9.jpg
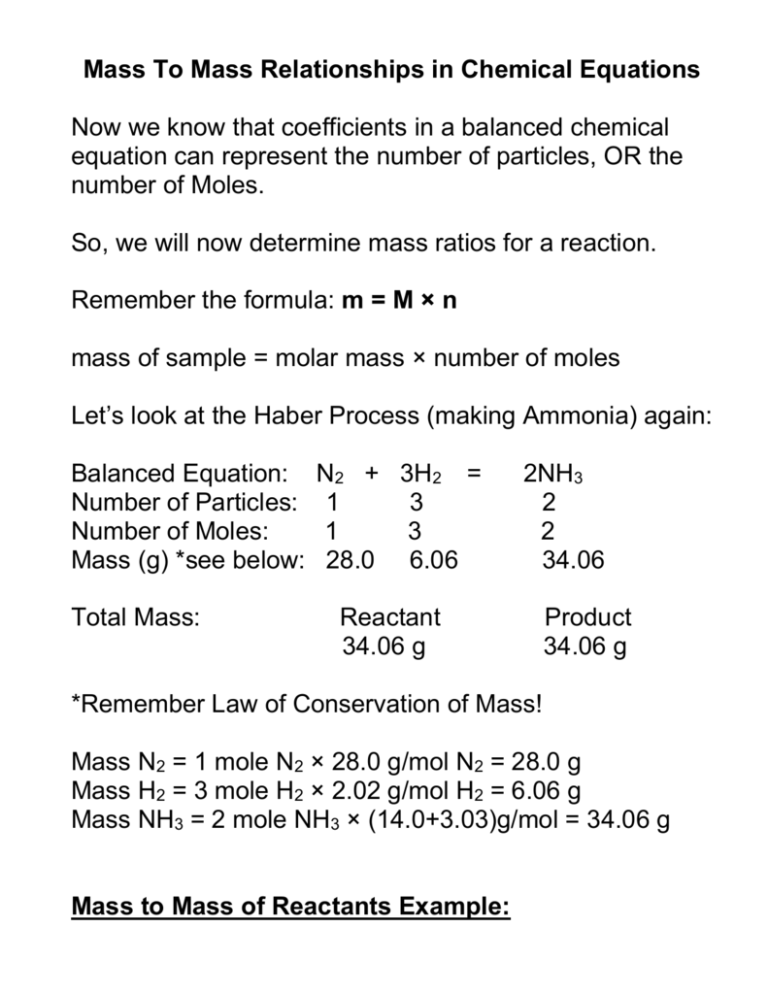
Mass To Mass Stoichiometry
https://s3.studylib.net/store/data/009037666_1-90282412b2cc0b631a5e5bb3b9eb3c6c-768x994.png
Chemistry 802 Mass Mass Stoichiometry Problems and Percent Yield Instructions Before viewing an episode download and print the note taking guides worksheets and lab data sheets for that episode keeping the printed sheets in order by page number During the lesson watch and listen for instructions to take notes pause the video complete Google Classroom You might need Calculator Periodic table Table salt or sodium chloride can be decomposed into sodium metal and chlorine gas by running electricity through molten NaCl A Downs cell produces sodium and chlorine from molten sodium chloride The balanced equation for this reaction is 2 NaCl l 2 Na l Cl A 2 g
This answer may then be converted to grams of SO 3 10 7 7 mol SO3 80 07gSO3 1 molSO3 862gSO3 10 7 7 m o l S O 3 80 07 g S O 3 1 m o l S O 3 862 g S O 3 The final answer is expressed to three significant figures Thus in a two step process we find that 862 g of SO 3 will react with 3 59 mol of Fe 2 O 3 Solution This is a two step conversion first using concentration as a conversion factor to determine the number of moles and then the molar mass of NaOH 40 0 g mol to convert to mass 0 765 L 1 93 molNaOH Lsolution 40 0gNaOH 1 molNaOH 59 1gNaOH 0 765 L 1 93 m o l N a O H L s o l u t i o n 40 0 g N a O H 1 m o l N a O H 59 1

Mass To Mass Stoichiometry Worksheet
https://db-excel.com/wp-content/uploads/2019/09/gas-stoichiometry-worksheet-answer-key-1-749x970.png

Stoichiometry Calculation Practice Worksheets
https://i.pinimg.com/originals/88/ed/77/88ed773f9843526af59f494501d1eb47.jpg
Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers - How do you find mass using molar mass If the moles of a substance are known the mass can be determined by multiplying the number of moles by the molar mass of the substance What Is