Unit Chemical Reactions Writing Formula Equations Ws 1 Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules
One dozen methane molecules and two dozen oxygen molecules react to yield one dozen carbon dioxide molecules and two dozen water molecules One mole of methane molecules and 2 moles of oxygen molecules react to yield 1 mole of carbon dioxide molecules and 2 moles of water molecules Step 1 Write each formula and balance each formula using SUBSCRIPTS Step 2 Balance the overall equation using coefficients 1 sulfur oxygen sulfur dioxide 2 zinc sulfuric acid zinc sulfate hydrogen 3 hydrogen nitrogen ammonia 4 hydrogen chlorine
Unit Chemical Reactions Writing Formula Equations Ws 1

Unit Chemical Reactions Writing Formula Equations Ws 1
http://www.worksheeto.com/postpic/2012/11/types-of-chemical-reactions-worksheet-answer-key_243740.png

17 Chemical Reactions Worksheet With Answers Worksheeto
https://www.worksheeto.com/postpic/2011/07/classifying-chemical-reactions-worksheet-answers_236692.jpg
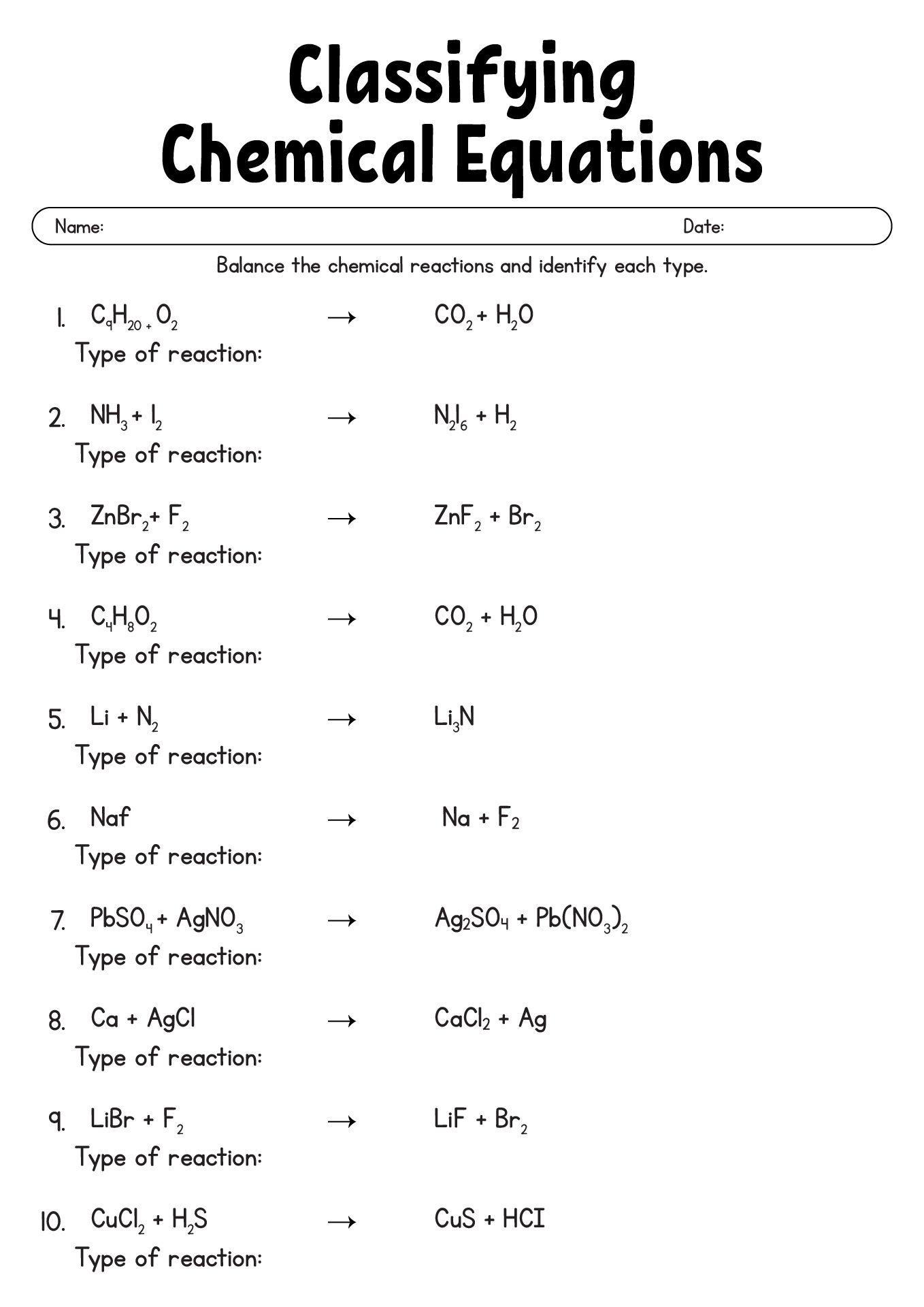
17 Chemical Reactions Worksheet With Answers Worksheeto
https://www.worksheeto.com/postpic/2011/07/chemical-reaction-types-worksheet_236693.png
PROBLEM 5 1 1 7 5 1 1 7 A novel process for obtaining magnesium from sea water involves several reactions Write a balanced chemical equation for each step of the process The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide The second step is the formation of Chemistry library 20 units 54 skills Unit 1 Atoms compounds and ions Unit 2 More about atoms Unit 3 More about molecular composition Unit 4 Mass spectrometry Unit 5 Chemical reactions and stoichiometry Unit 6 More about chemical reactions Unit 7 Electronic structure of atoms Unit 8 Periodic table
If a fractional coefficient has been used multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced Example 7 4 1 7 4 1 Combustion of Heptane Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint
More picture related to Unit Chemical Reactions Writing Formula Equations Ws 1
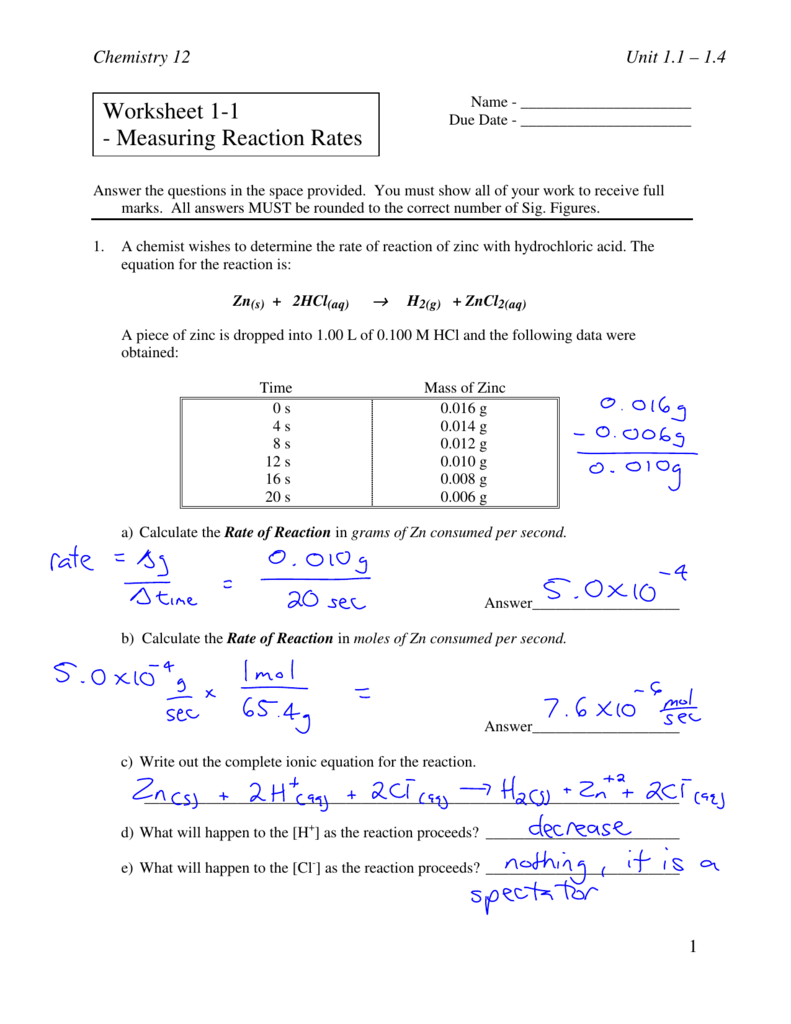
Unit 7 Reaction Equations Worksheet 1 Equations Worksheets
https://www.equationsworksheets.net/wp-content/uploads/2022/07/chemistry-unit-7-reaction-equations-worksheet-1-answers.png

What Is A Chemical Equation Definition And Examples
https://sciencenotes.org/wp-content/uploads/2021/10/What-Is-a-Chemical-Equation-768x512.png
Chemical Reactions And Chemical Equations Owlcation
https://images.saymedia-content.com/.image/t_share/MTc0NjM5MTYzODk3OTQ4MTUw/chemical-reactions-and-chemical-equations.jpg
Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water 6 From the statement nitrogen gas and hydrogen gas react to produce ammonia gas identify the reactants and the products 7 Balance NaClO3 NaCl O2 8 Balancing Equations A balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter It may be confirmed by simply summing the numbers of atoms on either side of the arrow and comparing these sums to
4 1 4 2 2 4 4 4 yes O 2 2 4 1 2 2 1 4 4 4 yes A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection Consider as an example the decomposition of water to yield molecular hydrogen and oxygen Balancing Equations A balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter It may be confirmed by simply summing the numbers of atoms on either side of the arrow and comparing these sums to

What Is A Chemical Reaction Overview Examples Expii
https://d20khd7ddkh5ls.cloudfront.net/general_chemistry_reaction_equation.png

Balancing Chemical Equation Worksheet 1
http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-36.jpg
Unit Chemical Reactions Writing Formula Equations Ws 1 - UNIT 6 CHEMICAL REACTIONS Date Agenda Homework Wed 1 18 Balancing and identifying reaction types Read p 203 210 Worksheet 1 Thurs 1 19 Review Worksheet 5 Write net ionic equations due tomorrow Wed 2 1 Review for test questions on review Study for test Thurs 2 2 Test Chemical Reactions 2