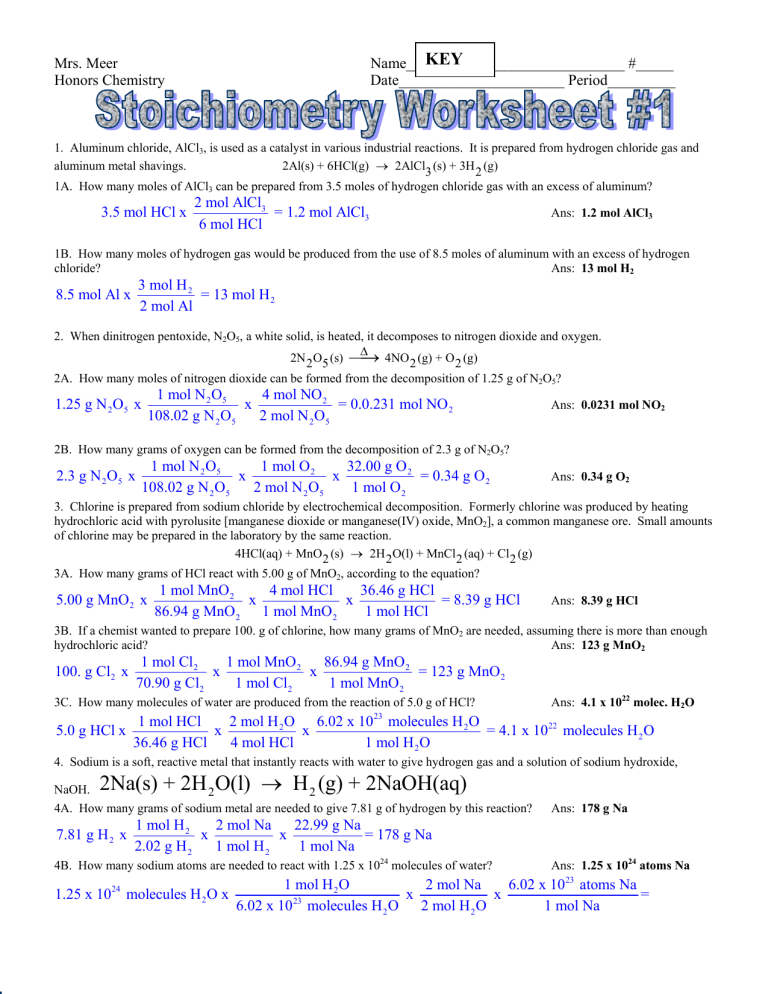
Stoichiometry Worksheet 1 Mass Mass A How many moles of dinitrogen tetrahydride are required to produce 57 moles of nitrogen B How many moles of dinitrogen tetroxide are required to produce 57 moles of nitrogen C How many moles of water are produced when 57 moles of nitrogen are made 3 Calculate the mass of aluminum oxide produced when 3 75 moles of aluminum burn in oxygen
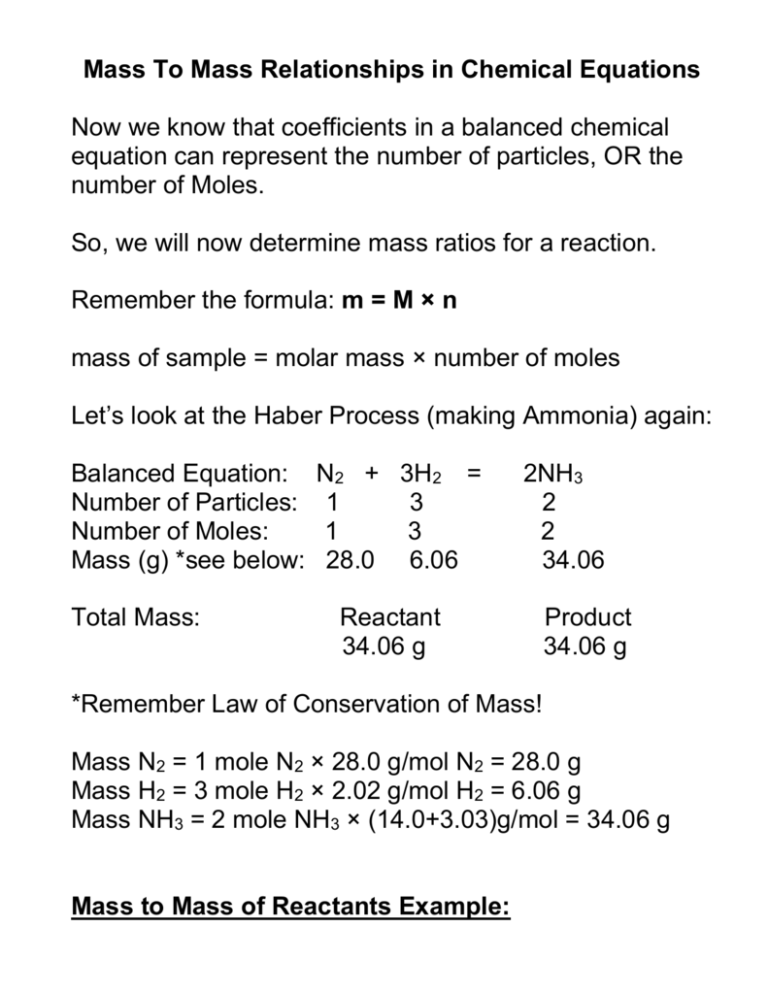
Example 12 4 1 12 4 1 Mass Mass Stoichiometry Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation NH4NO3 s N2O g 2H2O l NH 4 NO 3 s N 2 O g 2 H 2 O l In a certain experiment 45 7g 45 7 g of ammonium nitrate is decomposed Find the mass of each of the products formed Stoichiometry Mass Mass Problems 1 10 Return to Stoichiometry menu grams of NaOH Solution to b 2 The molar ratio between Na O that are consumed 1 Determine moles of Na 2 The molar ratio between Na 18 015 g H x x x 100 g H 61 979 g Na
Stoichiometry Worksheet 1 Mass Mass
Stoichiometry Worksheet 1 Mass Mass
https://s3.studylib.net/store/data/009037666_1-90282412b2cc0b631a5e5bb3b9eb3c6c-768x994.png

Stoichiometry 1 Mass Mass Relationships YouTube
https://i.ytimg.com/vi/VCXgpm-6r5M/maxresdefault.jpg

Stoichiometry Conversions Worksheet
https://i.pinimg.com/736x/e1/4f/8d/e14f8ddab451e7d637109f2ea4fe9619.jpg
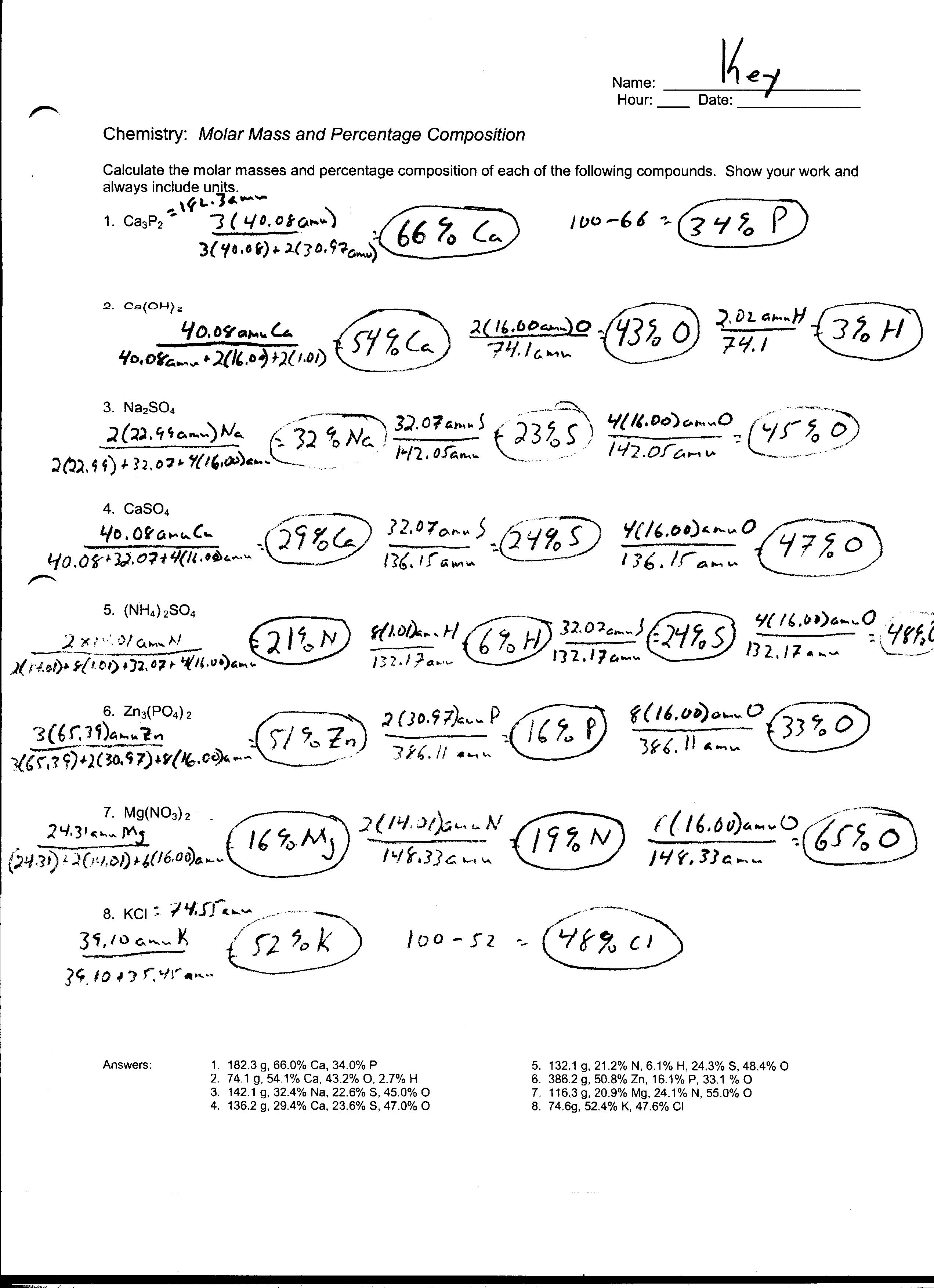
Example 1 How many grams of hydrogen gas are needed to react completely with 54 0 g of oxygen gas given the following unbalanced chemical reaction 1 Balance the chemical equation 2 Convert grams of the substance given 54 0 g 32 0 g mol 1 6875 mol of O Note the use of 32 0 and not 16 0 Based on a given mass of the substance calculate the mass of another substance using a balanced chemical equation Mole mole calculations are not the only type of calculation that can be performed using balanced chemical equations Recall that molar mass can be determined on the basis of a chemical formula and used as a conversion factor
G A x mole A 1 mole B molar mass A mole ratio from molar mass B the balanced equation Double lined boxes are Conversion Factors to convert from one quantity to another mole ratios reactant s and s y lar mass lar mass y lar mass Stoichiometry Practice Worksheet Author Ian Guch Subject Solution First let us work the problem in stepwise fashion We begin by converting the mass of CCl 4 to moles of CCl 4 using the molar mass of CCl 4 16 05 g mol as the conversion factor 100 0 g C H 4 1 m o l C H 4 16 05 g C H 4 6 231 m o l C H 4
More picture related to Stoichiometry Worksheet 1 Mass Mass

Unit 8 Stoichiometry Worksheet 1 Mole Relationships
https://s3.studylib.net/store/data/008807415_1-1b6d3416d419a4f5f0e3635301570c6e-768x994.png
Stoichiometry Worksheets And Key Answers
https://s1.studylib.net/store/data/008807410_1-90f9e6ac9b0dbf8ca0e0287b89ac7440-768x994.png
Mass Mass Problems Worksheet Stoichiometry Mass mass Problems
http://www.worksheeto.com/postpic/2013/12/mass-to-mole-stoichiometry-worksheet-answer-key_208083.jpg
To do this start by dividing the smallest number of moles into each of the numbers of moles of elements i e set the smallest number to 1 This may yield integers or it may yield decimal results that correspond closely to rational fractions For example 1 25 2 75 1 2 5 11 Sample Problem Mass Mass Stoichiometry Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation NH 4 NO 3 s N 2 O g 2 H 2 O l In a certain experiment 45 7 g of ammonium nitrate is decomposed Find the mass of each of the products formed Step 1 List the known quantities and plan the
Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C 4H 10 13 O 2 8 CO What mass of iron is needed to react with 16 0 grams of sulfur 27 87 g Fe b How many grams of FeS are produced When 1 20 mole of ammonia reacts the total number of moles of products formed is Honors Chemistry 1B Stoichiometry Worksheet 1 Name Use Stoichiometry to solve the following problems Use the conversion chart above to help 1 Magnesium reacts with hydrochloric acid according to the following chemical equation Mg s HCl aq MgCl2 aq H2 g

Stoichiometry Worksheet 1 Mass Mass Answer Key
https://db-excel.com/wp-content/uploads/2019/09/gas-stoichiometry-worksheet-answer-key-1-749x970.png

Mass Mass Problems Worksheet Stoichiometry Mass mass Problems
https://s3.studylib.net/store/data/007166444_1-7a531d61bd1991f401a61c5d393074cc.png
Stoichiometry Worksheet 1 Mass Mass - Home Courses Chemistry 215 Engelhardt Topic 16 Stoichiometry Mass to Mass Conversions Wksht 1 Stoichiometry Mass to Mass Conversions Wksht 1 Converting mass to mass Click LT 1 Mass Mass Conversions wkst 1 doc link to view the file Video Tutorial on Stoichiometry from Khan Academy