Stoichiometry Problems Chem Worksheet 12 2 Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction
Chemistry Stoichiometry Problem Sheet 2 Directions Solve each of the following problems Show your work including proper units to earn full credit CaCl2 AgNO3 Ca NO3 2 AgCl How many grams of silver chloride are produced when 45 g of calcium chloride react with excess silver nitrate Stoichiometry Practice Worksheet Solve the following stoichiometry grams grams problems Using the following equation 2 NaOH H2S04 21 120 Na2S04 How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid
Stoichiometry Problems Chem Worksheet 12 2

Stoichiometry Problems Chem Worksheet 12 2
https://i2.wp.com/images.sampletemplates.com/wp-content/uploads/2016/11/17162152/Gas-Stoichiometry-Worksheet.jpg
Stoichiometry Problems Chem Worksheet 12 2 Answer Key Ivuyteq
https://lh5.googleusercontent.com/proxy/hDNxIRC-aW5zVSJSp8nJQHyifmLHS2V-g4lgVha74Jq1BfBk2Ha-0Iw9izAGIHFFyUdjBU78P7hf4vHaU_s0bx8tmztMF7wE2OdEZxcNLTqXnLzOxbXjzsee27ZD1uv0=s0-d
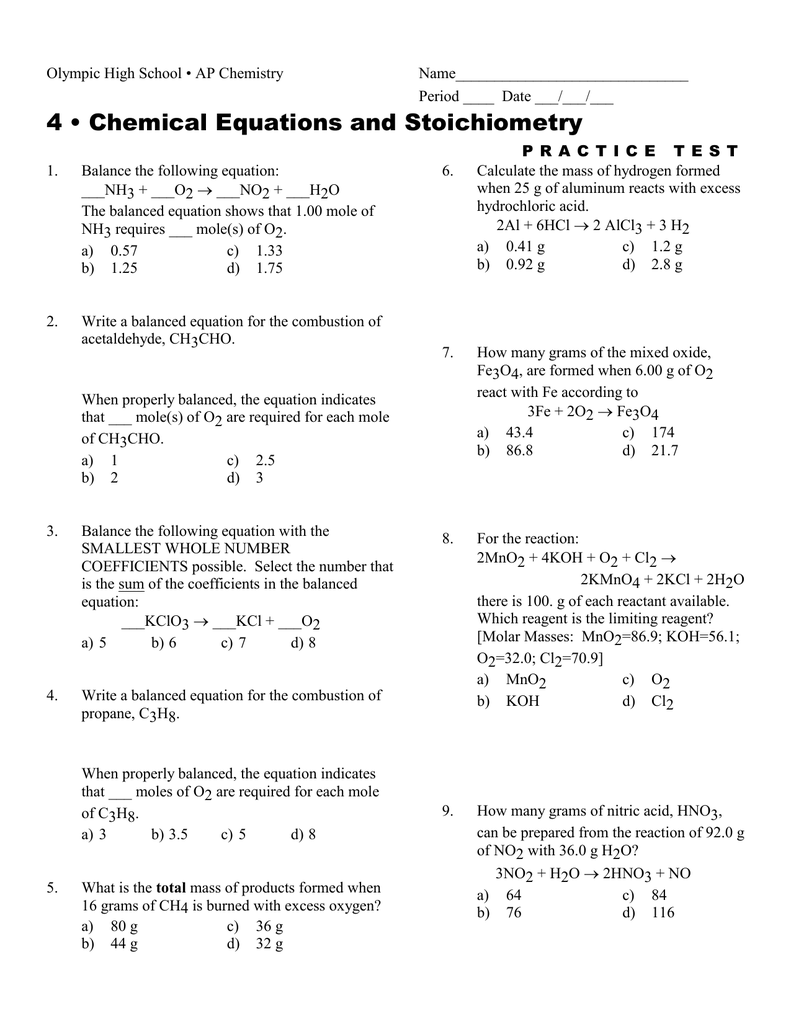
Chemical Equations And Stoichiometry Worksheet
https://s2.studylib.net/store/data/015569690_1-aa5c7dc453e2ca870609462b0e57f952.png
Solve the following stoichiometry grams grams problems 6 Using the following equation 2 NaOH H2SO4 2 H2O Na2SO4 How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl
Ideal stoichiometry How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess Stuck Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class Solution A In any stoichiometry problem the first step is always to calculate the number of moles of each reactant present In this case we are given the mass of K 2 Cr 2 O 7 in 1 mL of solution which we can use to calculate the number of moles of K 2 Cr 2 O 7 contained in 1 mL moles K2Cr2O7 1 mL 0 25 mg K2Cr2O7 mL 1 g 1000 mg 1
More picture related to Stoichiometry Problems Chem Worksheet 12 2

13 Chemistry Stoichiometry Worksheet Answer Key Worksheeto
https://www.worksheeto.com/postpic/2012/10/stoichiometry-worksheet-answer-key_245020.png

Chemical Equations And Stoichiometry Worksheet
https://i.pinimg.com/originals/4e/01/ca/4e01cac725d484b960225a0aca6c2e91.png

Chemistry Stoichiometry Worksheet
https://s3.studylib.net/store/data/008548962_1-d4995c2d6d093dffee5a0f6aad055019.png
This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula Stoichiometrty Practice Problems PSI Chemistry Name Classwork Set 1 1 2C2H6 7O2 4CO2 6H2O a How many moles of O2 are required to react with 24 moles of C2H6 b How many grams of C2H6 are required to react with 12 moles of O2 c How many grams of O2 are required to react with 200g of C2H6
Answers 1A 30 mol Ag 1B 30 mol AgNO3 1C 20 mol H2O 1D 10 mol NO 2A 38 mol N2H4 2B 19 mol N2O4 2C 76 mol H2O 3 191 g Al2O3 B How many moles of aluminum oxide are made if 3580 g of manganomanganic oxide are consumed C How many moles of manganomanganic oxide will react with 5 33 x 1025 atoms of aluminum D 1 Fermentation is a complex chemical process of making wine by converting glucose into ethanol and carbon dioxide C6H12O6 s 2 C2H5OH l 2 CO2 g A Calculate the mass of ethanol produced if 500 0 grams of glucose reacts completely 500 0 g C6H12O6 1 mol C H 6 12
Reaction Stoichiometry Worksheet
https://lh6.googleusercontent.com/proxy/M8R3veLE9E44YwwmUBs608FQtjAKUtv2AplyfllgJv1zqOm9dor_aaGAQxiZxILywnzQN6me7505zLLDDDeV-5K0P4EoWk-BvbifmsmDz8R2dpDBS1lJG3u_jD3wRMPd45GdpIyQ=w1200-h630-p-k-no-nu
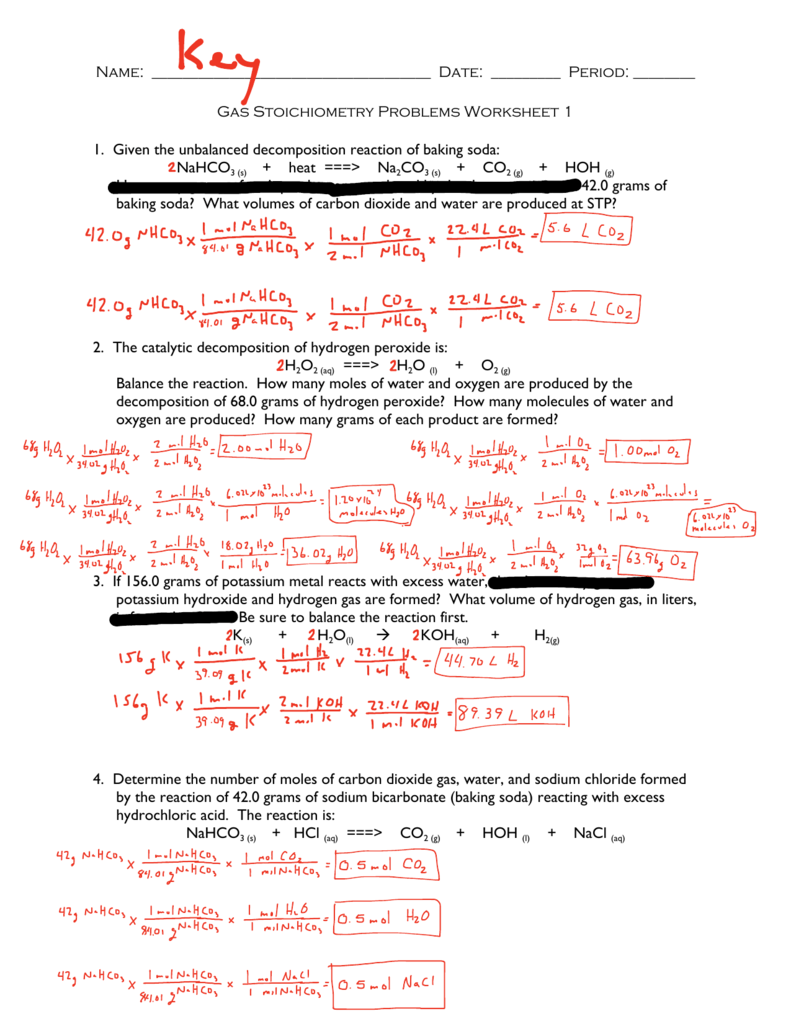
Stoichiometry Worksheet Answer Key
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png
Stoichiometry Problems Chem Worksheet 12 2 - Ideal stoichiometry How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess Stuck Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class