Stoichiometry Practice Problems Worksheet Stoichiometry Problem Sheet 1 Name Hour Date acid react according to the following balanced equation B From the amount of nitric acid given in Part A how many moles of silver nitrate will be produced C From the amount of nitric acid given in Part A how many moles of water will be produced D
Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction The following flow chart may help you work stoichiometry problems Remember to pay careful attention to what you are given and what you are trying to find Fermentation is a complex chemical process of making wine by converting glucose into ethanol and carbon dioxide C6H12O6 s 2 C2H5OH l 2 CO2 g
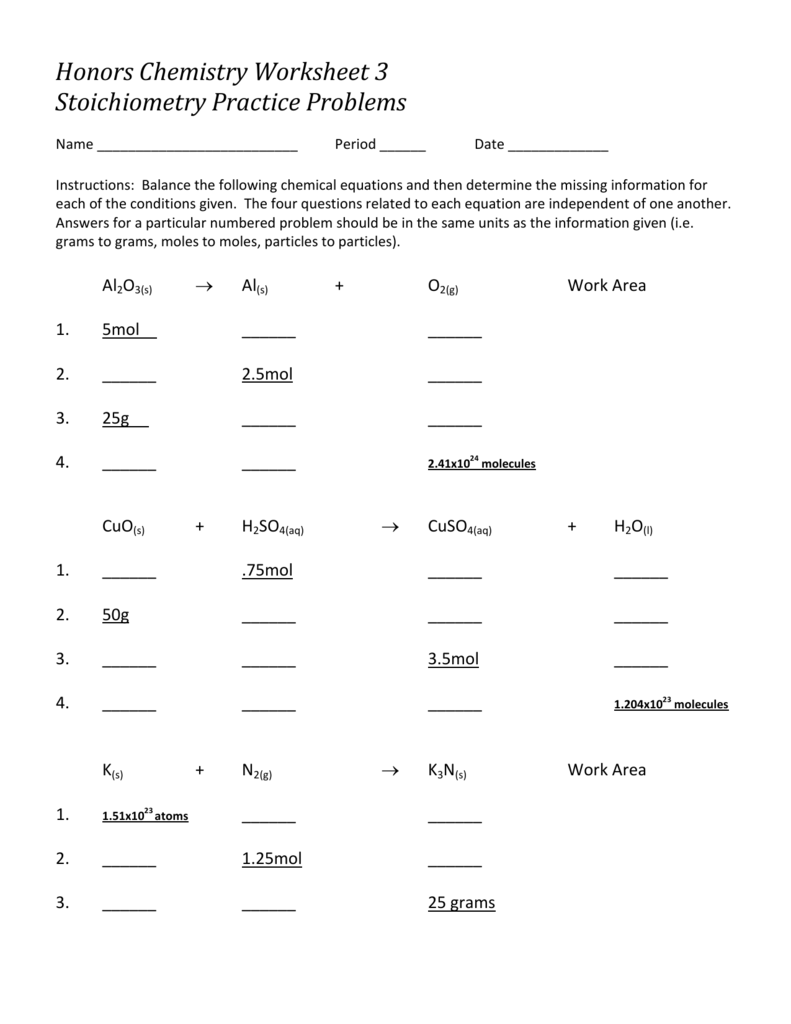
Stoichiometry Practice Problems Worksheet
Stoichiometry Practice Problems Worksheet
https://s3.studylib.net/store/data/008351429_1-690533bd35cd021cad902f8855cbaa92.png

Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008351423_1-c38642ce11131619171b8ee313df5e1d-768x994.png

Stoichiometry Practice Problems Printable Pdf Download
https://data.formsbank.com/pdf_docs_html/83/838/83806/page_1_thumb_big.png
A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl Google Classroom You might need Calculator Periodic table Given the following reaction Zn CuCl A 2 ZnCl A 2 Cu How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess moles round to three significant figures Show Calculator Show Periodic Table Stuck
Stoichiometry questions Google Classroom One type of anaerobic respiration converts glucose C 6 H 12 O 6 to ethanol C 2 H 5 O H and carbon dioxide If the molecular weight of glucose is 180 grams mol and the molar mass of ethanol is 46 g mol how many grams of carbon dioxide are produced when 1 mol of glucose is digested via respiration Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry Balance the following equations 1 N2 F2 NF3 2 C6H10 O2 CO2 H2O 3 HBr KHCO3 H2O KBr CO2 4 GaBr3 Na2SO3 Ga2 SO3 3 NaBr 5 SnO NF3 SnF2 N2O3
More picture related to Stoichiometry Practice Problems Worksheet

Stoichiometry Problems Worksheet 1 Answers Worksheets
https://i.pinimg.com/originals/3d/f7/d3/3df7d3b6b0b0b9e87f1ebb771a4ed1cb.jpg

10 Stoichiometry Practice Worksheet
https://i2.wp.com/s3.studylib.net/store/data/009765137_1-c6fa1d72307309453a1b6cc6193da43d.png
Worksheet 11 1 11 2 Stoichiometry Practice Problems Google Docs
https://lh5.googleusercontent.com/bDFKt2PeeGqM24L0ulAvQqtAsCY_YiF0SV-DLPMqOlRspauMKcp_dAawrThMMvYWupXoX-c0VAS-LQ=w1200-h630-p
KEY Solutions for the Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N2 3 F2 2 NF3 2 2 C6H10 17 O2 12 CO2 10 H2O 3 4 5 1 HBr 1 KHCO3 1 H2O 1 KBr 1 CO2 2 GaBr3 3 Na2SO3 1 Ga2 SO3 3 6 NaBr 3 SnO 2 NF3 3 SnF2 1 N2O3 Q4 Given the following reaction H2SO4 Na2CO3 Na2SO4 H2O CO2 H 2 S O 4 N a 2 C O 3 N a 2 S O 4 H 2 O C O 2 Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40 0 mL of H2SO4 H 2 S O 4 to neutralize 46 7 mL of a 0 364 M Na2CO3 N a 2 C O 3 solution
1 Calculate the number of moles of NaOH that are needed to react with 500 0 g of H2SO4 according to the following equation H2SO4 2 NaOH Na2SO4 2 H2O ANS 10 19 mol 2 Calculate the mass of NH3 that can be produced from the reaction of 125 g of NCl3 according to the following equation NCl3 3 H2O NH3 3 HOCl Stoichiometrty Practice Problems PSI Chemistry Name Classwork Set 1 1 2C2H6 7O2 4CO2 6H2O a How many moles of O2 are required to react with 24 moles of C2H6 b How many grams of C2H6 are required to react with 12 moles of O2 c How many grams of O2 are required to react with 200g of C2H6

Stoichiometry Worksheet 1 Mass Mass
https://images.sampletemplates.com/wp-content/uploads/2016/11/17162322/Mass-to-Mass-Stoichiometry-Worksheet.jpg

Stoichiometry Worksheet 2 Answers
https://i.pinimg.com/originals/46/04/a8/4604a8eb773a060ac9777f7367ea71fb.png
Stoichiometry Practice Problems Worksheet - Google Classroom You might need Calculator Periodic table Given the following reaction Zn CuCl A 2 ZnCl A 2 Cu How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess moles round to three significant figures Show Calculator Show Periodic Table Stuck