Stoichiometry Practice Problems Pdf Stoichiometrty Practice Problems PSI Chemistry Name Classwork Set 1 1 2C2H6 7O2 4CO2 6H2O a How many moles of O2 are required to react with 24 moles of C2H6 b How many grams of C2H6 are required to react with 12 moles of O2 c How many grams of O2 are required to react with 200g of C2H6
Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction Stoichiometry with Solutions Problems Stoichiometry with Solutions Name 1 H3PO4 3 NaOH Na3PO4 3 H2O How much 0 20 M H3PO4 is needed to react with 100 ml of 0 10 M NaOH 2 2 HCl Zn ZnCl2 H2 When you use 25 ml of 4 0 M HCl to produce H2 gas how many grams of zinc does it react with
Stoichiometry Practice Problems Pdf
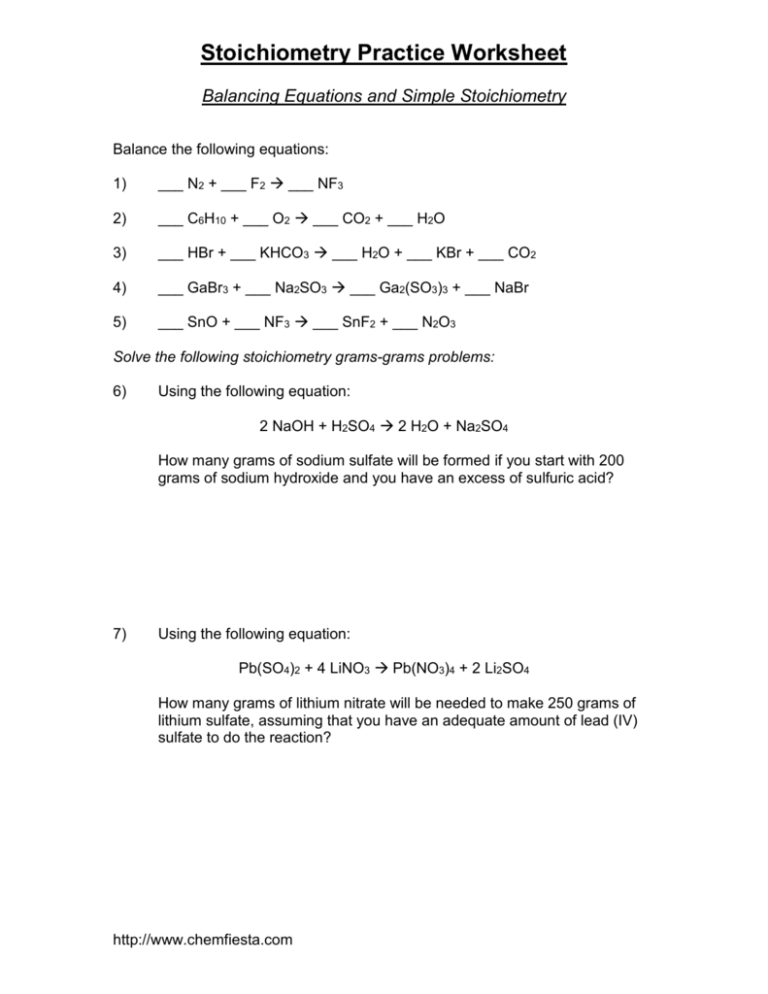
Stoichiometry Practice Problems Pdf
https://s3.studylib.net/store/data/008602372_1-31aac1f4045a4e526277821c8002b456-768x994.png

Stoichiometry Practice Problems Worksheet Answers Pdf
https://i2.wp.com/images.sampletemplates.com/wp-content/uploads/2016/11/17162152/Gas-Stoichiometry-Worksheet.jpg
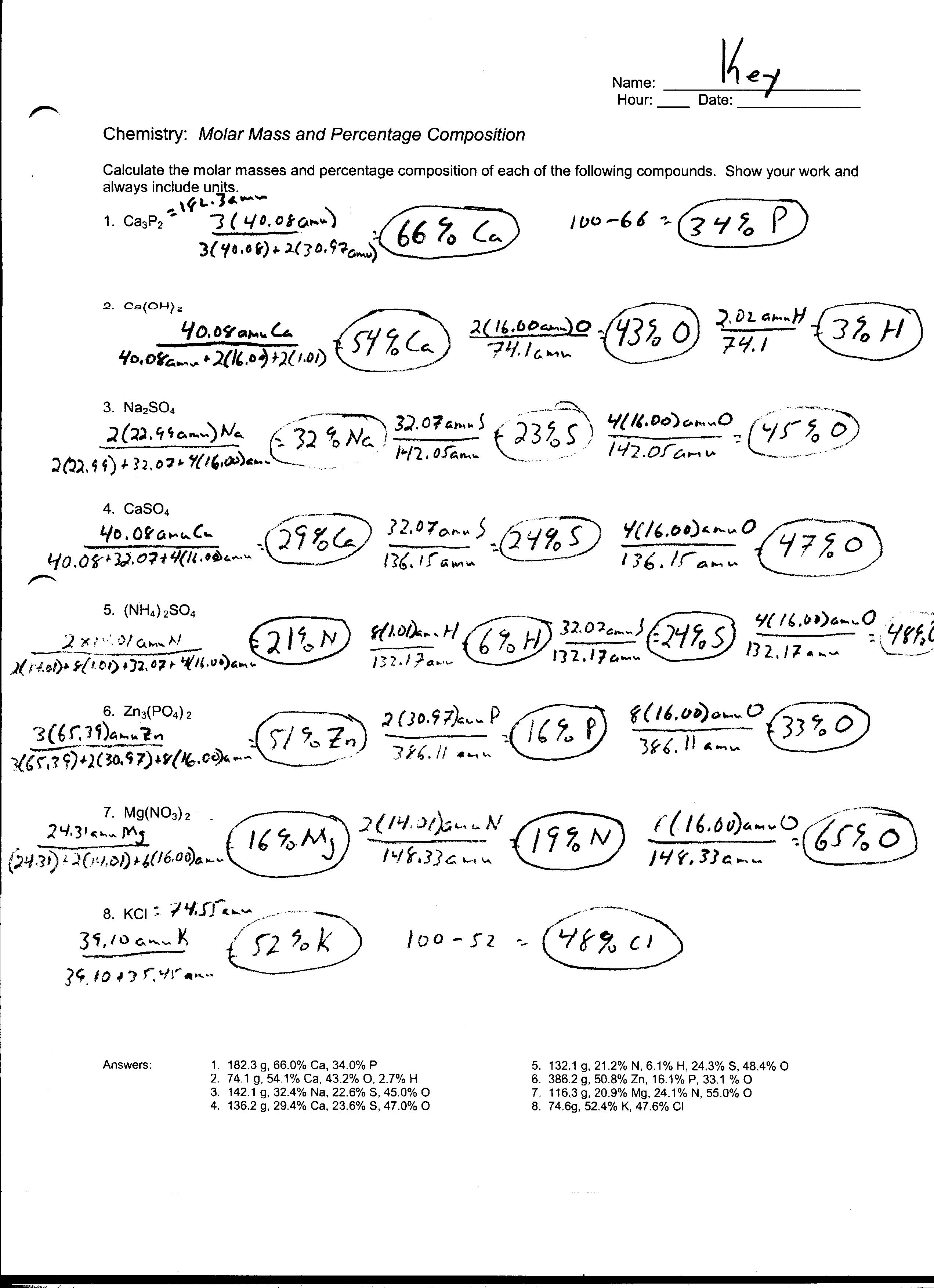
Mass Mass Problems Worksheet Stoichiometry Mass mass Problems
http://www.worksheeto.com/postpic/2013/12/mass-to-mole-stoichiometry-worksheet-answer-key_208083.jpg
This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula Answer the following stoichiometry related questions 12 Write the balanced equation for the reaction of acetic acid with aluminum hydroxide to form water and aluminum acetate 13 Using the equation from problem 12 determine the mass of aluminum acetate that can be made if I do this reaction with 125 grams of acetic acid
1 Calculate the number of moles of NaOH that are needed to react with 500 0 g of H2SO4 according to the following equation H2SO4 2 NaOH Na2SO4 2 H2O ANS 10 19 mol 2 Calculate the mass of NH3 that can be produced from the reaction of 125 g of NCl3 according to the following equation NCl3 3 H2O NH3 3 HOCl The following flow chart may help you work stoichiometry problems Remember to pay careful attention to what you are given and what you are trying to find Fermentation is a complex chemical process of making wine by converting glucose into ethanol and carbon dioxide C6H12O6 s 2 C2H5OH l 2 CO2 g
More picture related to Stoichiometry Practice Problems Pdf

Stoichiometry Problems Part 2 YouTube
https://i.ytimg.com/vi/oPn0HjMS2K4/maxresdefault.jpg
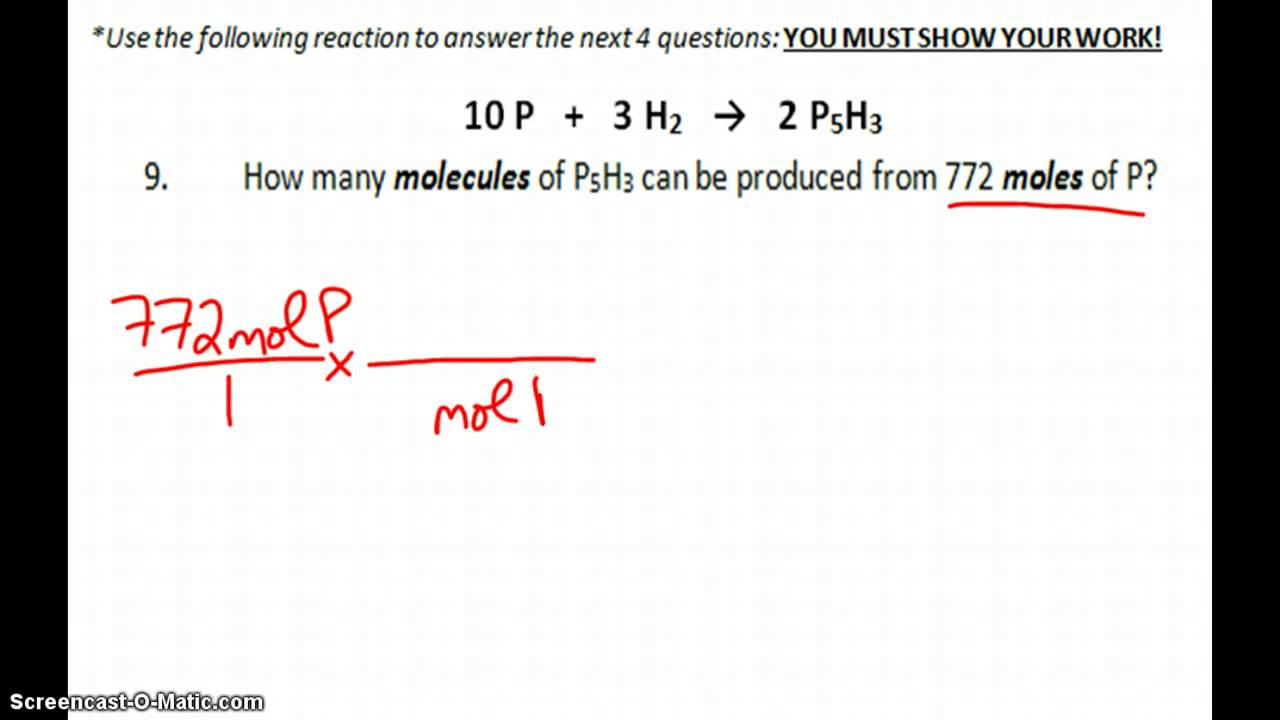
Stoichiometry Practice Test Answers YouTube
https://i.ytimg.com/vi/CCelcRQbuSY/maxresdefault.jpg
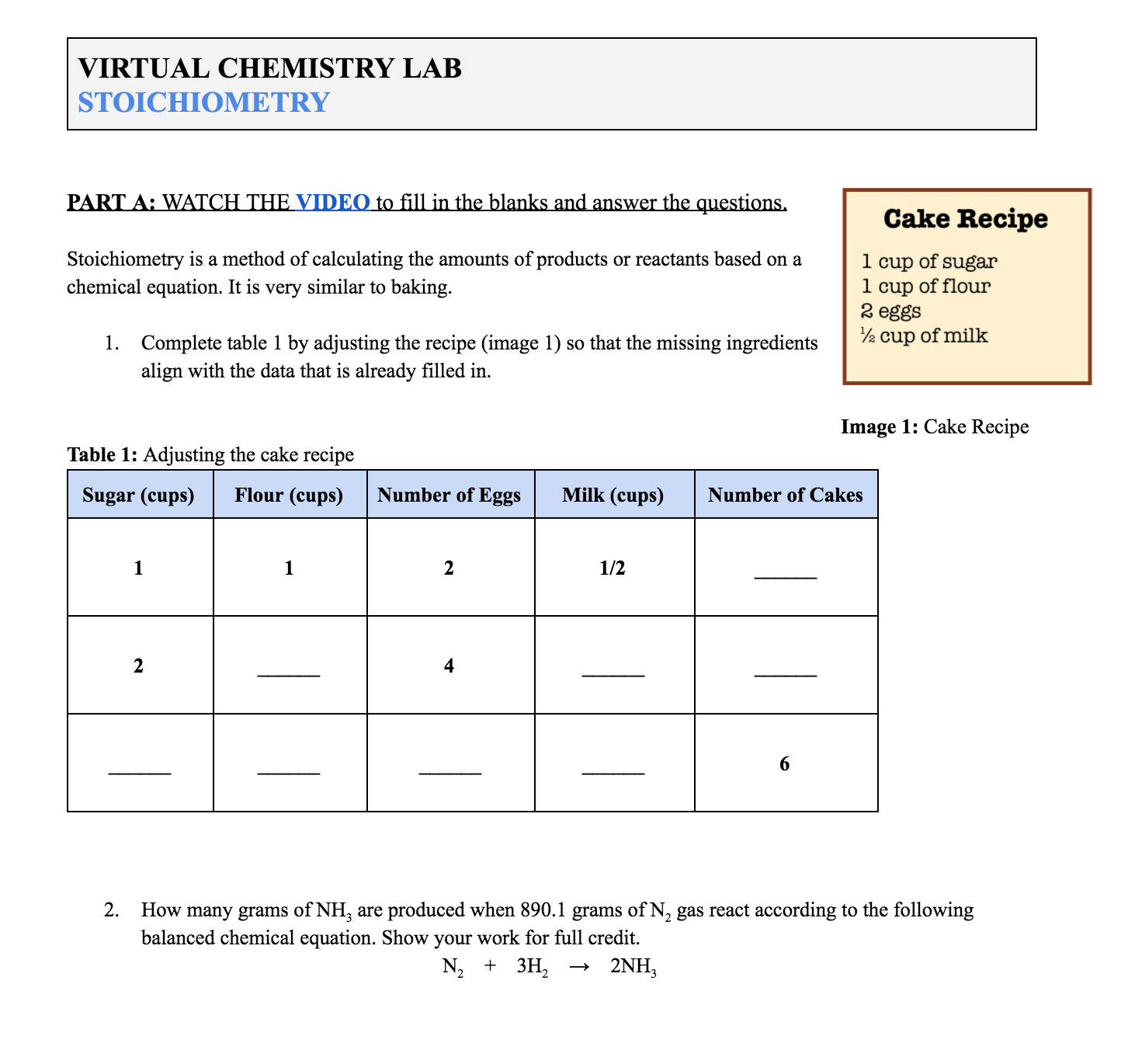
Stoichiometry A Free Virtual Chemistry Lab Activity Chemical
https://www.chemedx.org/sites/www.chemedx.org/files/stoichlab2.png
Chemistry Problems Stoichiometry Nigel Freestone chem textbook Stiochiometry Practice Problems a Determine the number of moles of N 2O 4 needed to react completely with 3 62 moles of N 2H 4 for the reaction 2N 2H 4 l N 2O 4 l 3N 2 g 4H 2O l b What mass of iron II sulfide can be formed from 14 0 g of sulphur Chapter 3 Stoichiometry Michigan State UniversityLearn the basic concepts and calculations of stoichiometry the quantitative study of chemical reactions in this introductory chemistry course from MSU Download the PDF lecture notes and practice with exercises and examples Explore how stoichiometry can be applied to real world problems such as environmental issues industrial processes
Ideal stoichiometry How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess Stuck Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class Question 10 Identify the energy graphs A and B as being for an endothermic or exothermic reaction A B Answer 4 7 Unit 4 Practice Problems is shared under a not declared license and was authored remixed and or curated by LibreTexts
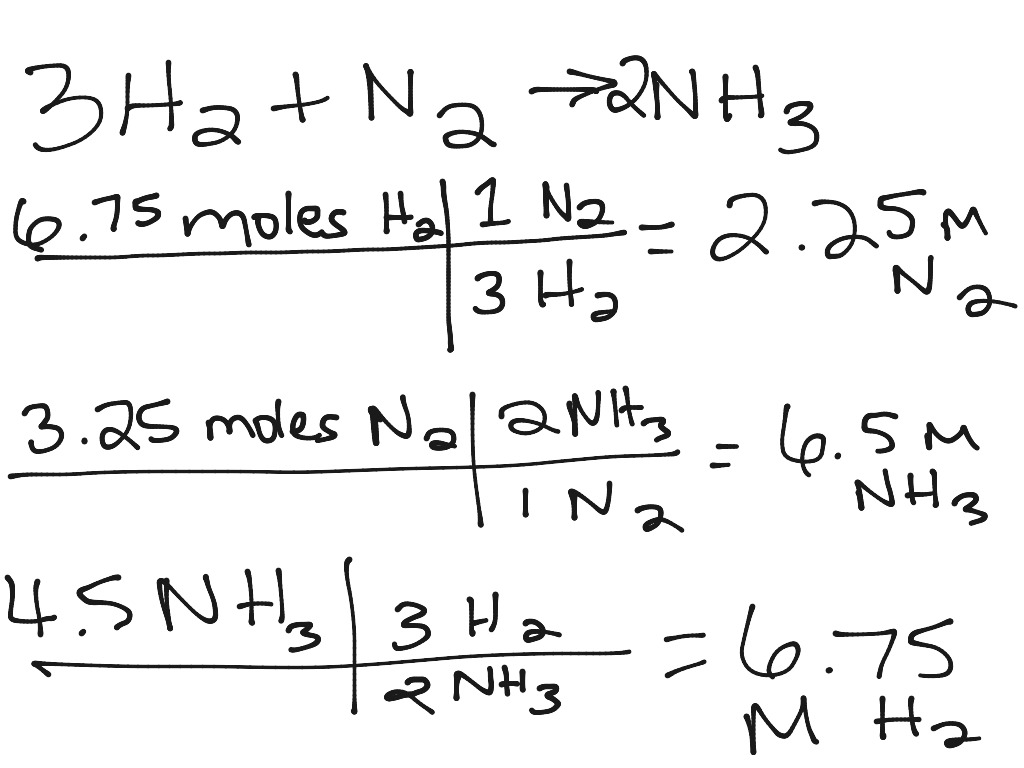
ShowMe Stoichiometry Practice
https://showme0-9071.kxcdn.com/files/595636/pictures/thumbs/1338494/last_thumb1389807736.jpg
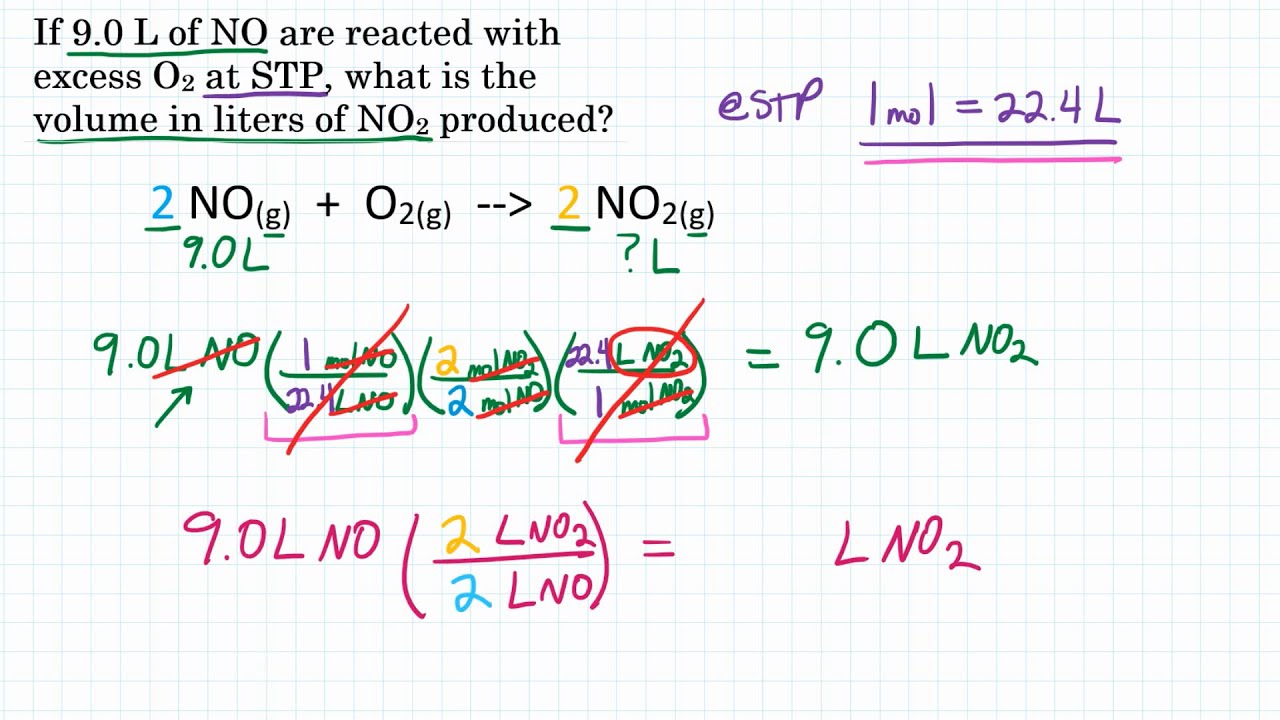
Ideal Gas Stoichiometry Volume To Volume Practice 1 YouTube
https://i.ytimg.com/vi/auF_HSWv0nE/maxresdefault.jpg
Stoichiometry Practice Problems Pdf - The following flow chart may help you work stoichiometry problems Remember to pay careful attention to what you are given and what you are trying to find Fermentation is a complex chemical process of making wine by converting glucose into ethanol and carbon dioxide C6H12O6 s 2 C2H5OH l 2 CO2 g