Stoichiometry Practice 2 Worksheet Answers This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula
Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction KEY Solutions for the Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N2 3 F2 2 NF3 2 2 C6H10 17 O2 12 CO2 10 H2O 3 4 5 1 HBr 1 KHCO3 1 H2O 1 KBr 1 CO2 2 GaBr3 3 Na2SO3 1 Ga2 SO3 3 6 NaBr 3 SnO 2 NF3 3 SnF2 1 N2O3
Stoichiometry Practice 2 Worksheet Answers
Stoichiometry Practice 2 Worksheet Answers
https://media.cheggcdn.com/media/c2f/c2f60bd7-885a-43bb-83c5-490efe502c37/image
Stoichiometry Worksheet Answers
https://imgv2-1-f.scribdassets.com/img/document/131383825/original/a396110907/1521550172?v=1
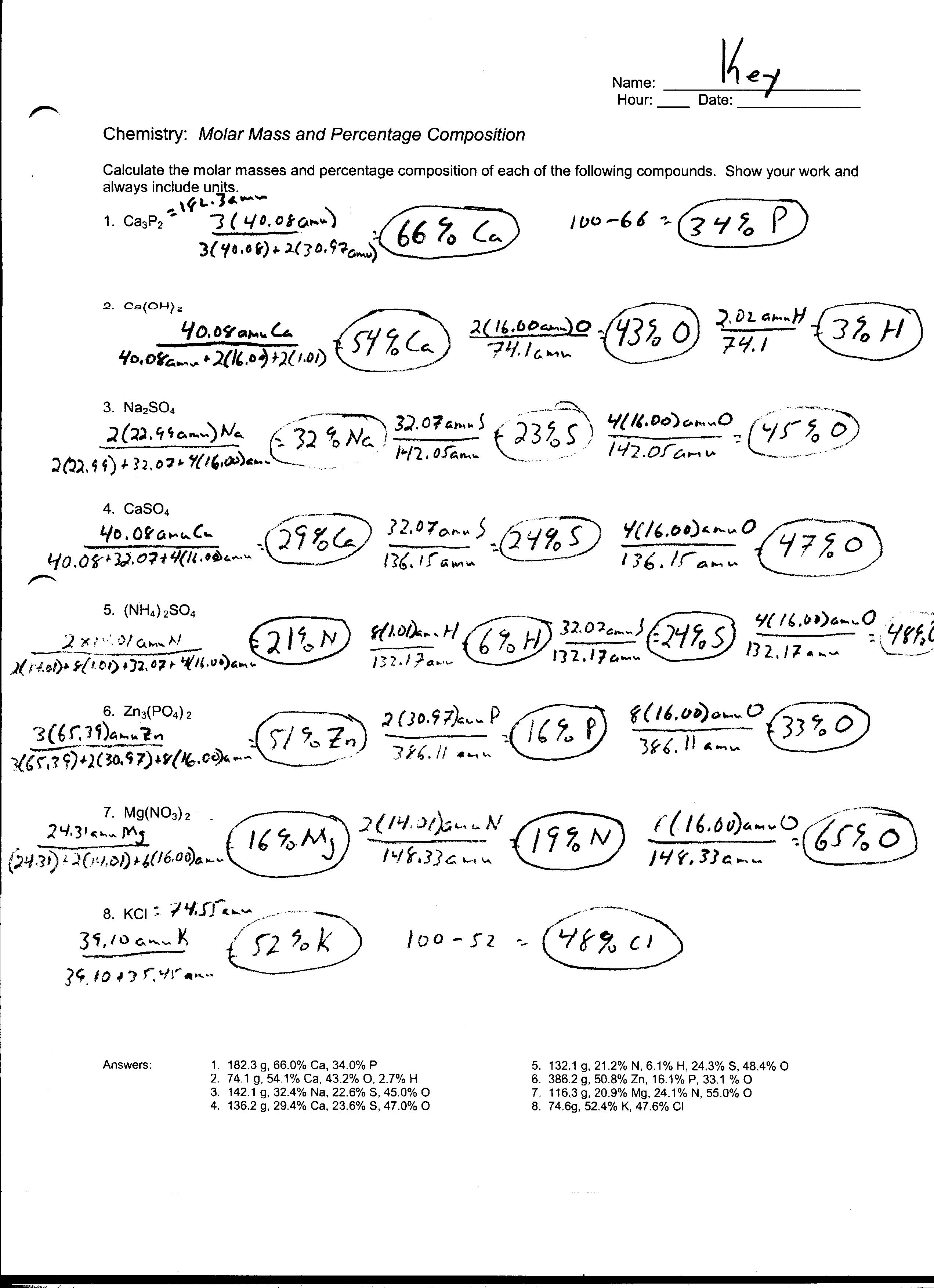
Mass Mass Problems Worksheet Stoichiometry Mass mass Problems
http://www.worksheeto.com/postpic/2013/12/mass-to-mole-stoichiometry-worksheet-answer-key_208083.jpg
A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl Answers 1 17 mL 2 3 3 g of zinc and 1 1 L of H 2 3 0 10L 4 5 3 L 5 2 0 x10 5 L 6 0 370 M Title Stoichiometry with Solutions Problems Author Dan Keywords solutions stoichiometry practice sheet Created Date
Google Classroom You might need Calculator Periodic table Given the following reaction Zn CuCl A 2 ZnCl A 2 Cu How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess moles round to three significant figures Show Calculator Show Periodic Table Stuck Stoichiometry Practice Worksheet Solve the following stoichiometry grams grams problems Using the following equation 2 NaOH H2S04 21 120 Na2S04 How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you have an excess of sulfuric acid
More picture related to Stoichiometry Practice 2 Worksheet Answers

Stoichiometry Lecture Answer Key Pdf Db excel
https://db-excel.com/wp-content/uploads/2019/09/stoichiometry-lecture-answer-key-pdf.jpg
Worksheet 11 1 11 2 Stoichiometry Practice Problems Google Docs
https://lh3.googleusercontent.com/8d7ELHV6YMszuHSQTLuJf0zqv9gJ0c-2EP--p9RA3Z_-wjZq_eq26aDE23Dfb4PKB7HrfyDBtg=w1200-h630-p
Solution Stoichiometry Chem Worksheet 15 6 Answer Key
https://imgv2-2-f.scribdassets.com/img/document/432466636/original/17fced1b61/1589396269?v=1
Q4 Given the following reaction H2SO4 Na2CO3 Na2SO4 H2O CO2 Calculate the molarity of the H2SO4 solution if it takes 40 0 mL of H2SO4 to neutralize 46 7 mL of a 0 364 M Na2CO3 solution Stoichiomet Practice Show pll work in your notebook including dimensional analysis and units 02 Fe203 a How many moles of iron would be needed to react with 3 82 moles of What is needed to react with 2 56 g of C 4H10 r S a C q HI b 3 10 H xo 0 42 L 2 3 a KC103 a KCI a When 62 0 g of potassium ch rate are reacted how
2 O 3 is used how many kilograms of iron can be produced The reaction is Fe 2 O 3 3 C 2 Fe 3 CO 9 The average human requires 120 0 grams of glucose C 6 H 12 O 6 per day How many grams of CO 2 in the photosynthesis reaction are required for this amount of glucose The photosynthetic reaction is 6 CO 2 6 H 2 O C 6 H 12 O Stoichiometry Practice Worksheet STOICHIOMETRY MAP FOR CHEMICAL REACTIONS Double lined boxes are Conversion Factors to convert from one quantity to another BALANCED CHEMICAL EQUATION REACTANTS PRODUCTS GIVEN grams molar mass MOLES WANTED grams molar mass xA GIVEN mole ratios reactant s and product s MOLES yB zC

Stoichiometric Conversions Worksheet
https://db-excel.com/wp-content/uploads/2019/09/gas-stoichiometry-worksheet-answer-key-1-749x970.png

Stoichiometry Problems Worksheet 1 Answers Worksheets
https://i.pinimg.com/originals/3d/f7/d3/3df7d3b6b0b0b9e87f1ebb771a4ed1cb.jpg
Stoichiometry Practice 2 Worksheet Answers - A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl


