Stoichiometry Map Problems 2 Stoichiometry Problem Sheet 2 KEY Chemistry Stoichiometry Problem Sheet 2 Directions Solve each of the following problems Show your work including proper units to earn full credit CaCl2 AgNO3 Ca NO3 2 AgCl
1 mol Fe 2 O 3 2 mol Al Using this ratio we could calculate how many moles of Al are needed to fully react with a certain amount of Fe 2 O 3 or vice versa In general mole ratios can be used to convert between amounts of any two substances involved in a chemical reaction Given the following reaction Zn CuCl A 2 ZnCl A 2 Cu How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess moles round to three significant figures Show Calculator Show Periodic Table Stuck
Stoichiometry Map Problems 2

Stoichiometry Map Problems 2
https://i.ytimg.com/vi/jkw8fBZbMc4/maxresdefault.jpg
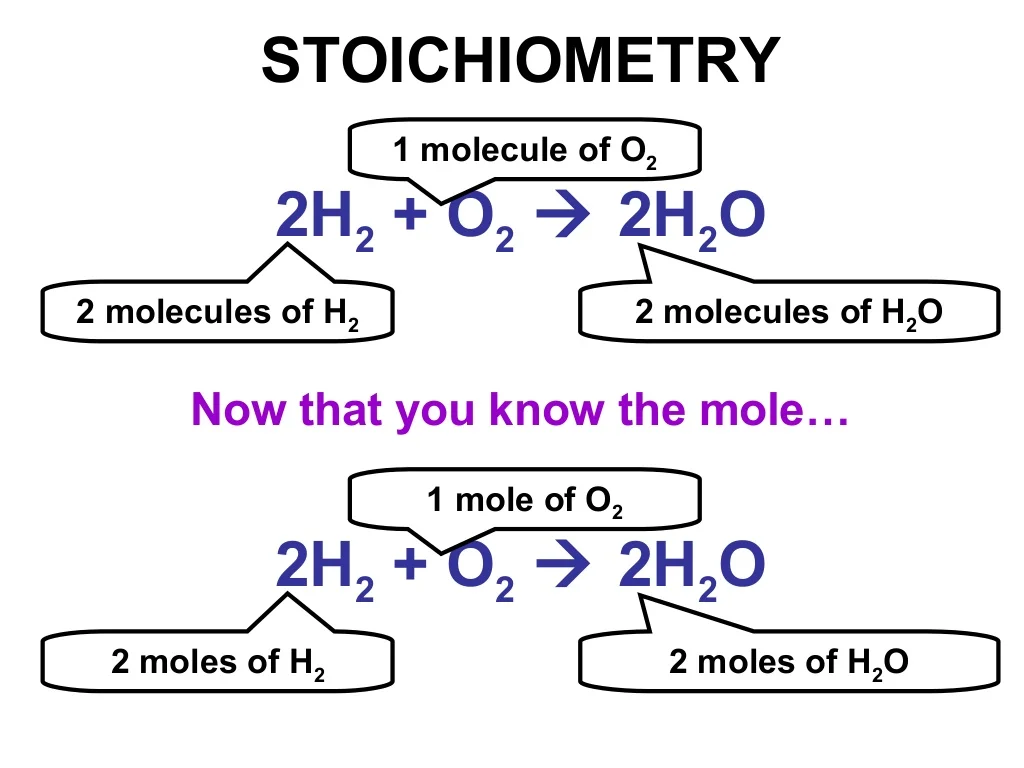
17 Stoichiometry
https://image.slidesharecdn.com/17stoichiometry-130220104619-phpapp02/95/17-stoichiometry-2-1024.jpg?cb=1361357227

Stoichiometric Conversions Worksheet
https://db-excel.com/wp-content/uploads/2019/09/gas-stoichiometry-worksheet-answer-key-1-749x970.png
Problems include a lot of scaffolding Includes 5 problems Problem Set ST7 Mass to Mass Stoichiometry 2 To use molar mass values and a balanced chemical equation to relate the mass of reactants to the mass of products Includes 5 problems Problem Set ST8 Mass to Mass Stoichiometry 3 A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl
Description Over the years I ve found this map complimentary worksheets and colored pencils are the BEST way for students to master 1 2 and 3 step stoichiometry problems Chapter 3 Stoichiometry Michigan State UniversityLearn the basic concepts and calculations of stoichiometry the quantitative study of chemical reactions in this introductory chemistry course from MSU Download the PDF lecture notes and practice with exercises and examples Explore how stoichiometry can be applied to real world problems such as environmental issues industrial processes
More picture related to Stoichiometry Map Problems 2
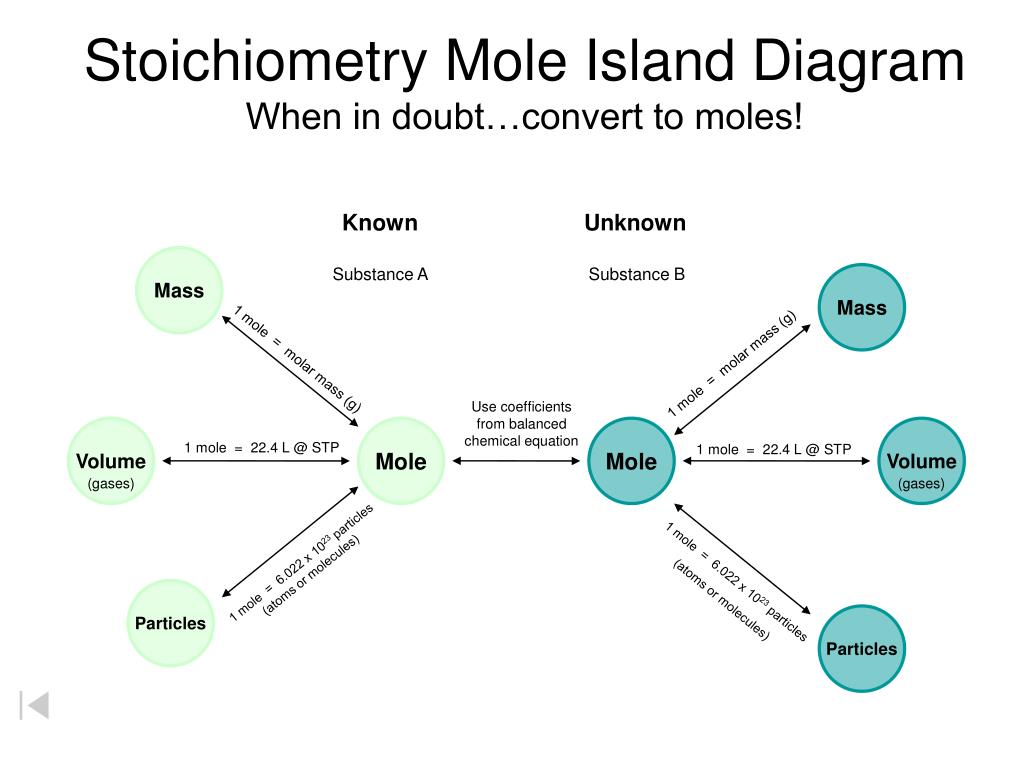
PPT Stoichiometry Mole Island Diagram When In Doubt convert To Moles
https://image.slideserve.com/640055/stoichiometry-mole-island-diagram-when-in-doubt-convert-to-moles-l.jpg

Mastering Stoichiometry Map Problems 2 Unlock The Answer Key
https://studyfinder.org/wp-content/pic/2248-stoichiometry-map-problems-2-answer-key.jpg
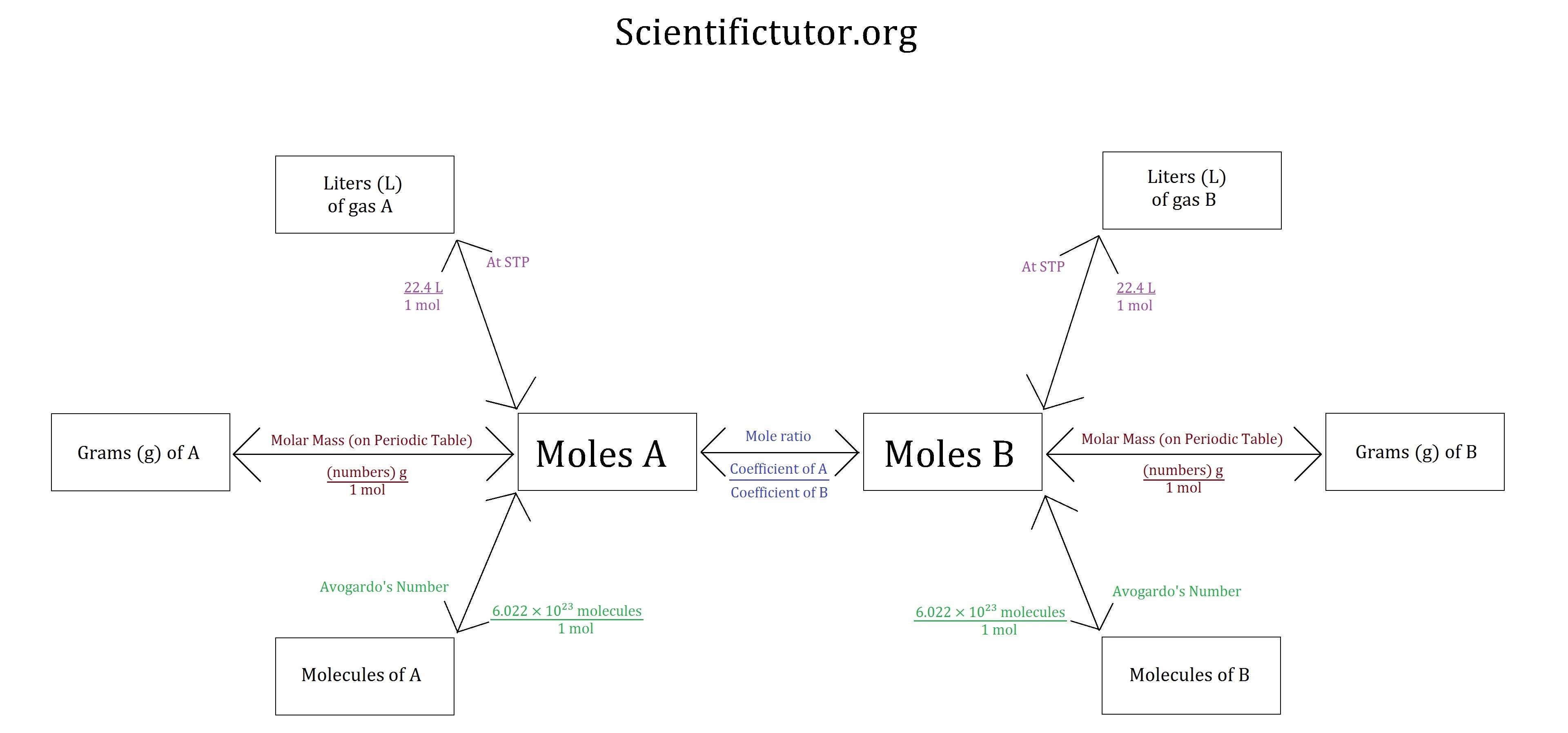
Chem Gas Stoichiometry Scientific Tutor
http://scientifictutor.org/wp-content/uploads/2016/10/Conversion-Diagram-STP-2.jpg
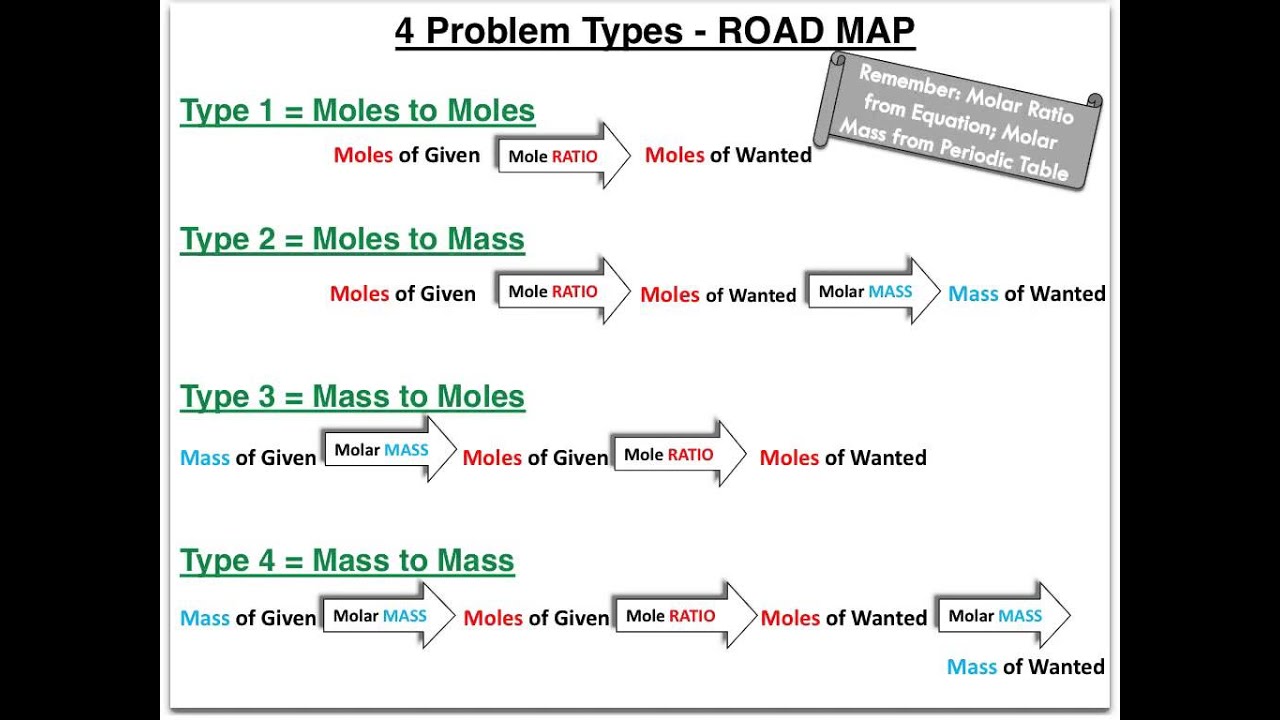
Using stoichiometry is a fundamental skill in chemistry that can be difficult for students to grasp This road map can be used as a tool to help students identify the steps needed to take to calculate 1 step 2 step and 3 step stoichiometry problems If a number is written on the road map ex 1 mol A or 1 L B that number does not change If Chemistry Stoichiometry Problem Sheet 2 KEY 9 2 24 2 2 23 2 2 2 2 4 63 x 10molecules I 1 mol I 6 02 x 10 moleculesI 1 mol Cl 1mol 71 g Cl Cl x 546 g Cl 10 292 g Ag 1 mol Ag 108 g Ag 1 mol Cu 1 mol Ag 63 5 g Cu 1 mol Cu x g Ag 86 g CuO 11 3 3 3 3 2 2 2 3 2 15 7 dmNH 1 mol NH 22 4 dmNH 1 mol Ca OH 2mol 74 g Ca OH 1 Ca OH x L 26 0 g Ca OH
Step 1 Write the chemical equation Step 2 Balance the chemical equation Step 3 Do any needed conversions You need SI units Step 4 Find the moles of the reactant s Use the stoichiometry road map Gas stoichiometry problems may require you to use the ideal gas law Step 5 Determine the limiting reagent Stoichiometry Practice Problems Problem 1 You mix of CaCl 2 into a solution with excess AgNO 3 hence CaCl 2 is your limiting reactant Assuming a 100 yield how much AgCl would you expect to produce in grams AgCl has a molecular weight of CaCl 2 and AgNO 3 react according to the following equation
Stoichiometry Explained YouTube
https://i.ytimg.com/vi/HeoSZo5EeAw/maxresdefault.jpg
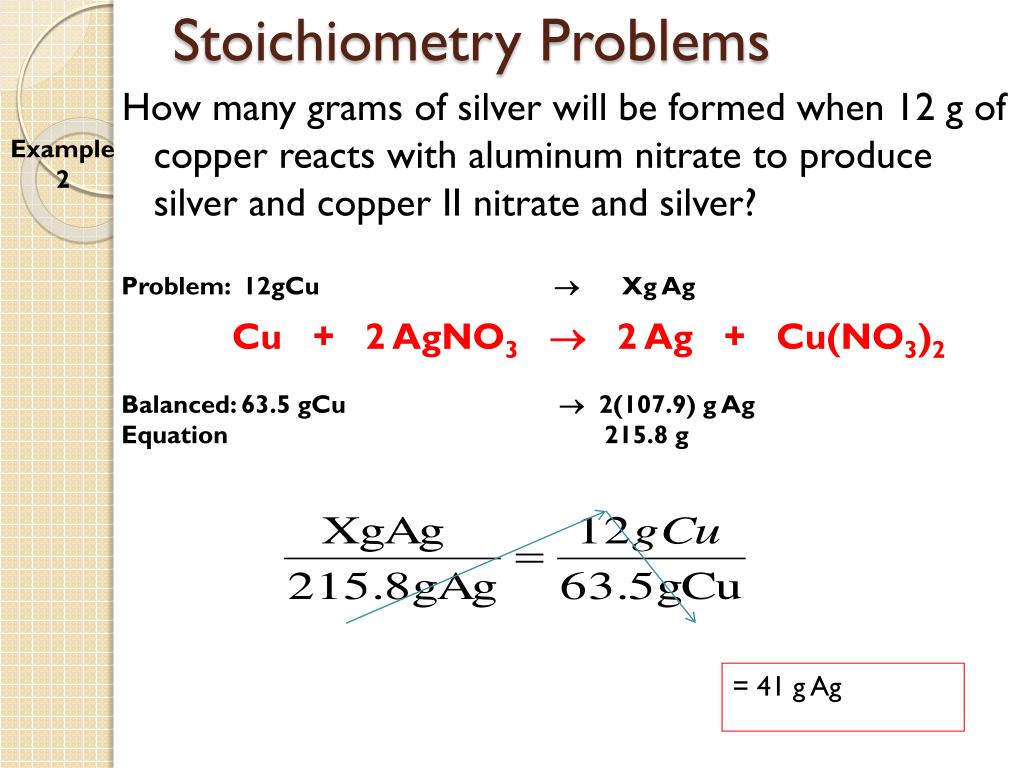
PPT Stoichiometry Mass Changes In Chemical Reactions PowerPoint
https://image1.slideserve.com/2012608/stoichiometry-problems1-l.jpg
Stoichiometry Map Problems 2 - Problems include a lot of scaffolding Includes 5 problems Problem Set ST7 Mass to Mass Stoichiometry 2 To use molar mass values and a balanced chemical equation to relate the mass of reactants to the mass of products Includes 5 problems Problem Set ST8 Mass to Mass Stoichiometry 3