Stoichiometry Homework 1 Answers A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl
Stoichiometry Worksheet 1 Answers 1 Given the following equation 2 C4H10 13 O2 8 CO2 10 H2O show what the following molar ratios should be a C4H10 O2 b O2 CO2 c O2 H2O d C4H10 CO2 e C4H10 H2O 2 Given the following equation 2 KClO3 2 KCl 3 O2 a Reaction Stoichiometry H 2 is produced by the reaction of 118 5 mL of a 0 8775 M solution of H 3 PO 4 according to the following equation 2Cr 2H3PO4 3H2 2CrPO4 2 Cr 2 H 3 PO 4 3 H 2 2 CrPO 4 Outline the steps necessary to determine the number of moles and mass of H 2 Answer Convert mL to L
Stoichiometry Homework 1 Answers
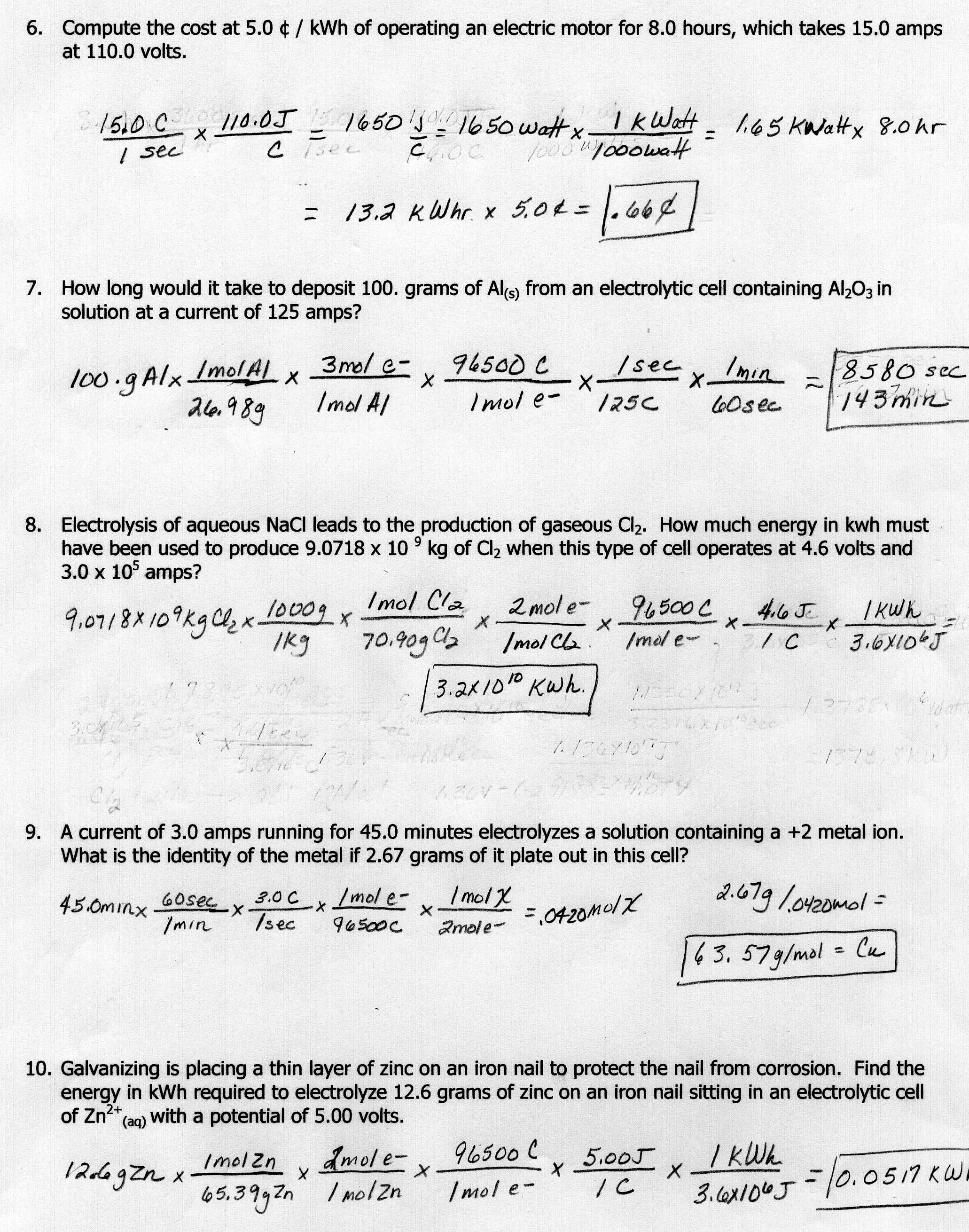
Stoichiometry Homework 1 Answers
http://www.worksheeto.com/postpic/2012/06/stoichiometry-worksheet-answers_670813.jpg

Mass Mass Stoichiometry Practice Worksheet Answers
https://i.pinimg.com/originals/88/ed/77/88ed773f9843526af59f494501d1eb47.jpg
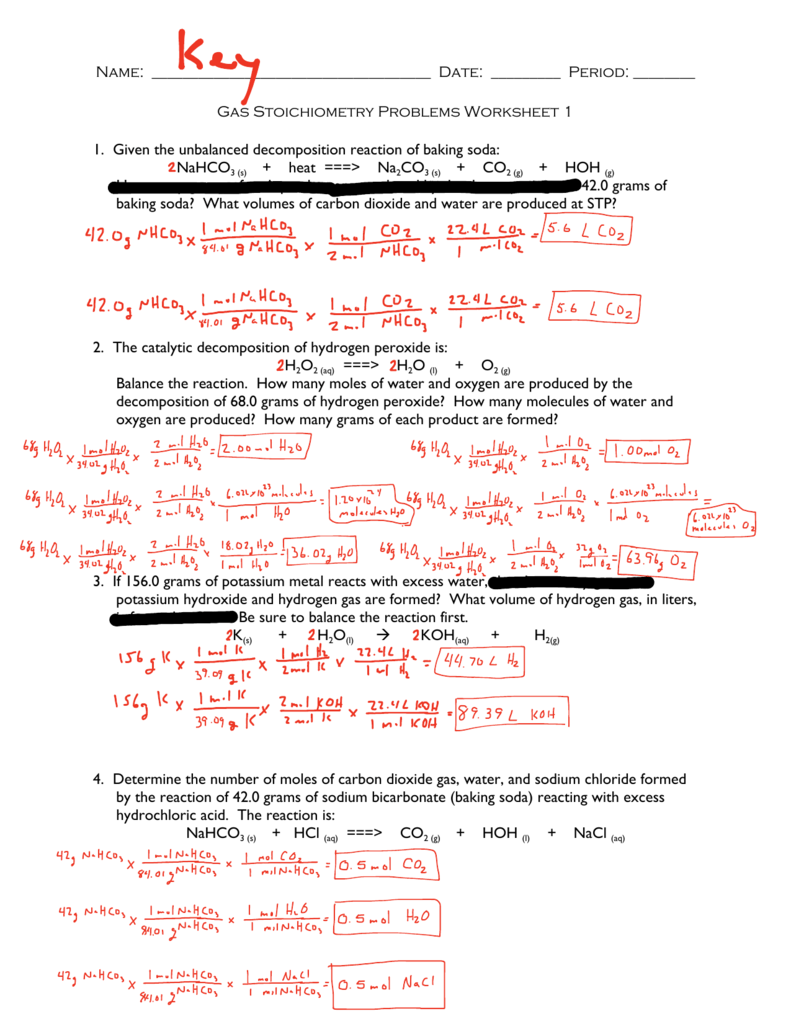
Stoichiometry Worksheet 1 Answer Key
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png
Stoichiometry WorkSheet 1 Worked Solutions Answer the following questions on your own paper Show all work Circle the final answer giving units and the correct number of significant figures 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H3PO4 Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction
1 The reaction of calcium hydride with water can be used to prepare small quantities of hydrogen gas as is done to fill weather observation balloons CaH2 s H2O I Ca OH 2 s H2 g not balanced a How many grams of water are consumed in the reaction of 56 2 g CaH2 CaH2 s 2H2O I Ca OH 2 s 2H2 g 56 2g CaH2 x 1 mol CaH2 Chemistry library 20 units 54 skills Unit 1 Atoms compounds and ions Unit 2 More about atoms Unit 3 More about molecular composition Unit 4 Mass spectrometry Unit 5 Chemical reactions and stoichiometry Unit 6 More about chemical reactions Unit 7 Electronic structure of atoms Unit 8 Periodic table
More picture related to Stoichiometry Homework 1 Answers
Stoichiometry Practice 2 Worksheet Answers Printable Word Searches
https://i2.wp.com/lh3.googleusercontent.com/proxy/YLfQO2Ktop3x54BhOh9tk13b7HDzDXRDMDkZ58yQ1ElhX3qHqEpxKSglUvtaUfhfmNx-mpIWj4NApaAlHN8bvPRDERlmJSpAcXXzhst3hzZG-KDFUVYdCrSlipnNYpAJ=w1200-h630-p-k-no-nu

Answer Key Stoichiometry Worksheet Answers
https://data.formsbank.com/pdf_docs_html/73/732/73239/page_1_thumb_big.png
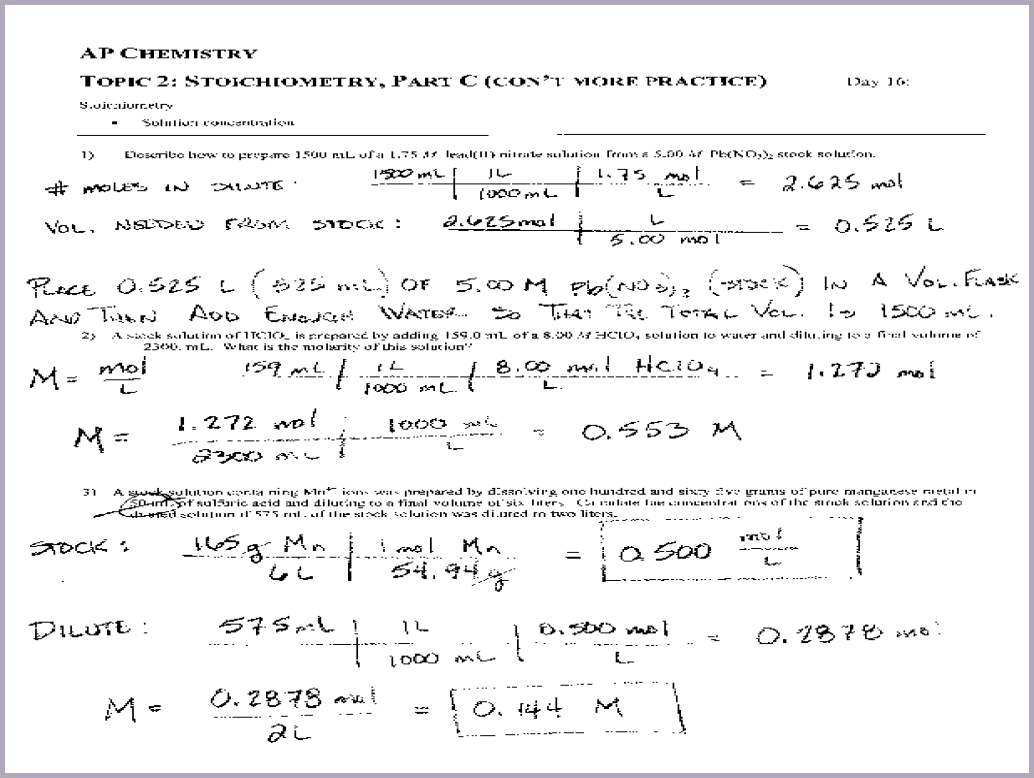
Stoichiometry Worksheet 1 Answers
https://www.unmisravle.com/wp-content/uploads/2018/03/chemistry_stoichiometry_worksheet_ap_chemistry_topic_2_1.png
Homework Set 1 1 2C3H7OH 9O2 6CO2 8H2O a How many moles of O2 are required to react with 58 moles of C3H7OH b How many moles of CO2 are required to react with 17 moles of O2 c How many grams of CO2 would be required to react with 7 8 moles of H2O d How many grams of C3H7OH are needed to produce 0 45 moles of water 1 mol Fe 2 O 3 2 mol Al Using this ratio we could calculate how many moles of Al are needed to fully react with a certain amount of Fe 2 O 3 or vice versa In general mole ratios can be used to convert between amounts of any two substances involved in a chemical reaction
Worksheet 4 Stoichiometry Concentrations and Reactions in Solutions 1 Stoichiometry 1 Mole to Mole Conversion Complete the table for the given reaction 2C2HC g 7O2 g 4CO2 g 6H2O g Adjust cells to your needs In the table below there are 7 independent questions Each starts with given number of moles of either reactant or product This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula
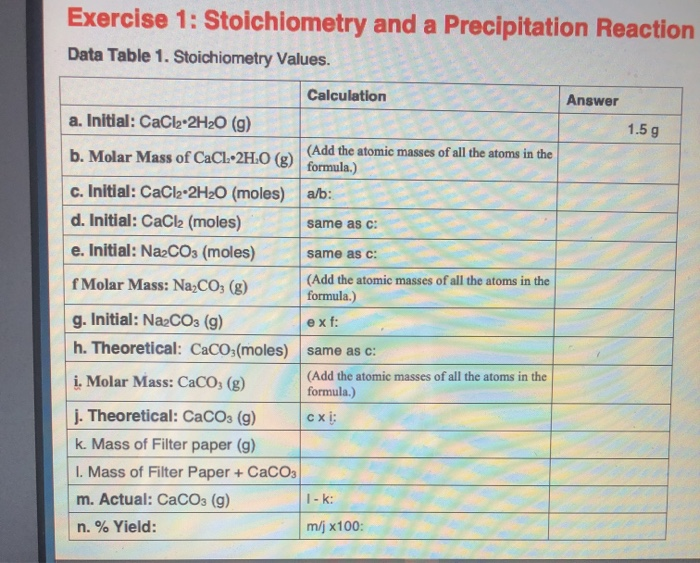
Solved Exercise 1 Stoichiometry And A Precipitation Chegg
https://media.cheggcdn.com/study/4db/4dbd8a8c-6d49-4905-bcd8-65f27aa93e0d/image.png
Solved Stoichiometry Worksheet 1 Mole to Mole Calculations Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/3b4/3b4d4cb8-6c97-40ce-bea8-08e407b09b2d/image
Stoichiometry Homework 1 Answers - Stoichiometry questions Google Classroom One type of anaerobic respiration converts glucose C 6 H 12 O 6 to ethanol C 2 H 5 O H and carbon dioxide If the molecular weight of glucose is 180 grams mol and the molar mass of ethanol is 46 g mol how many grams of carbon dioxide are produced when 1 mol of glucose is digested via respiration
