Stoichiometry 2 Worksheet Answers Stoichiometry Worksheet 2 Answer Key 1 a 2 13 b 13 8 c 13 10 d 2 8 or 1 4 e 2 10 or 1 5 2 The KClO3 O2 molar ratio is 2 3 2 mol KClO3 3 mol O2 12 00 mol KClO3 x 18 00 mol x 18 00 mol of O2 3 Given the following equation 2 K Cl2 2 KCl
Answers 1A 30 mol Ag 1B 30 mol AgNO3 1C 20 mol H2O 1D 10 mol NO 2A 38 mol N2H4 2B 19 mol N2O4 2C 76 mol H2O 3 191 g Al2O3 B How many moles of aluminum oxide are made if 3580 g of manganomanganic oxide are consumed C How many moles of manganomanganic oxide will react with 5 33 x 1025 atoms of aluminum D Write and balance the chemical equation How many moles of CO2 are required to make 120 0g of glucose 6H2O 6CO2 C6H12O6 6O2 4 000 mol CO2 5 2NaClO3 2NaCl 3O2 Balance and answer the following questions a How many grams of NaCl are produced when 20 00mol of NaClO3 react 1170g NaCl b
Stoichiometry 2 Worksheet Answers
Stoichiometry 2 Worksheet Answers
https://imgv2-2-f.scribdassets.com/img/document/432466636/original/17fced1b61/1589396269?v=1
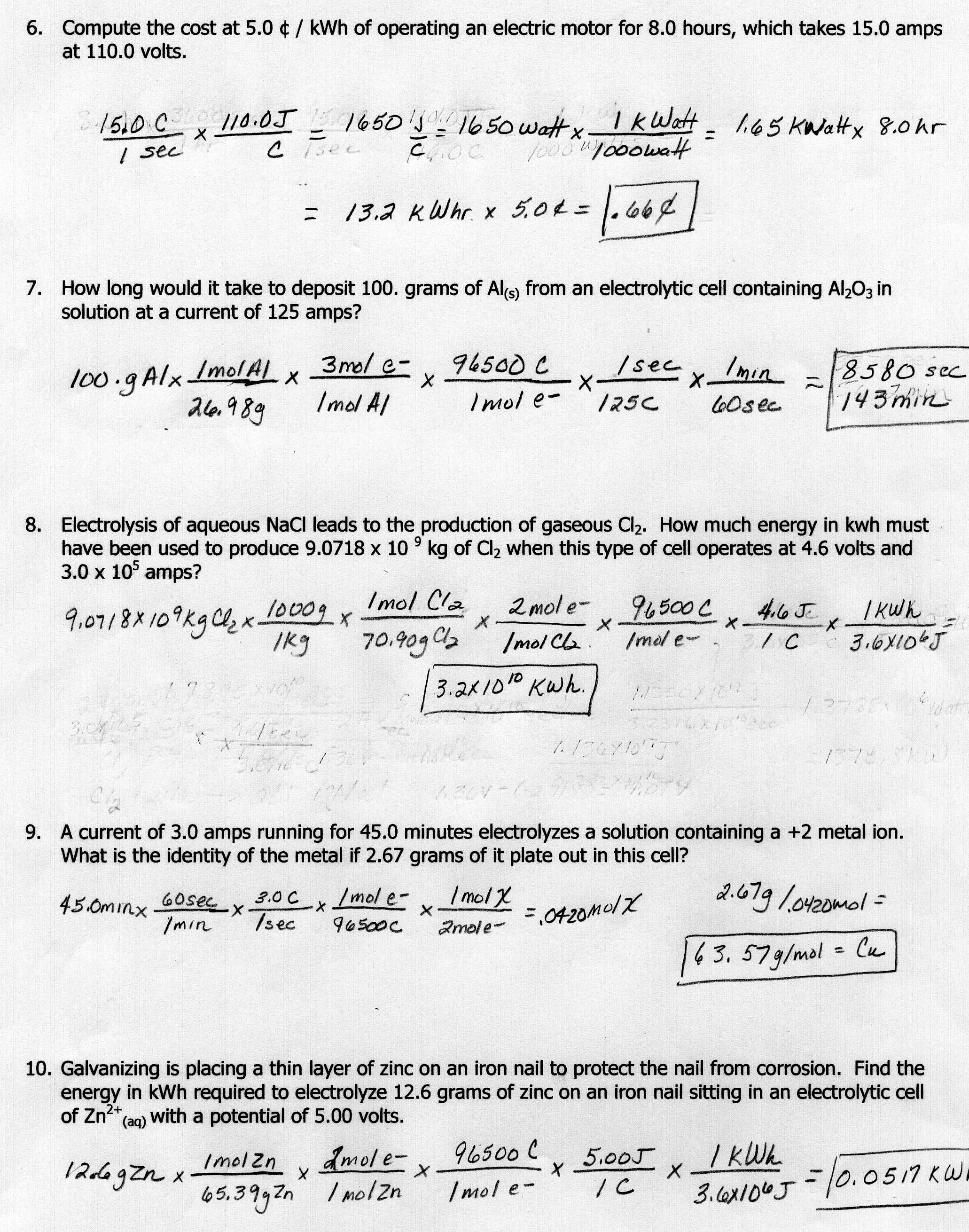
Stoichiometry Worksheet Answer Key
http://www.worksheeto.com/postpic/2012/06/stoichiometry-worksheet-answers_670813.jpg

Stoichiometry Calculation Practice Worksheets
https://i.pinimg.com/originals/88/ed/77/88ed773f9843526af59f494501d1eb47.jpg
Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following a 9 36 10 24 formula units of NaCl
KEY Solutions for the Stoichiometry Practice Worksheet Balancing Equations and Simple Stoichiometry KEY Balance the following equations 1 1 N2 3 F2 2 NF3 2 2 C6H10 17 O2 12 CO2 10 H2O 3 4 5 1 HBr 1 KHCO3 1 H2O 1 KBr 1 CO2 2 GaBr3 3 Na2SO3 1 Ga2 SO3 3 6 NaBr 3 SnO 2 NF3 3 SnF2 1 N2O3 Q4 Given the following reaction H2SO4 Na2CO3 Na2SO4 H2O CO2 H 2 S O 4 N a 2 C O 3 N a 2 S O 4 H 2 O C O 2 Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40 0 mL of H2SO4 H 2 S O 4 to neutralize 46 7 mL of a 0 364 M Na2CO3 N a 2 C O 3 solution
More picture related to Stoichiometry 2 Worksheet Answers

Stoichiometry Practice 2 Worksheet Answers Printable Word Searches
https://i2.wp.com/s3.studylib.net/store/data/025349974_1-ab754f5a90c0fe64b187b19524c5aea0-768x994.png

Unit Stoichiometry Mass Mass Calculations Worksheet 2 Answers Laabeja
https://i.redd.it/55y9udo3q5r21.jpg

Worksheet For Basic Stoichiometry Answer
https://chessmuseum.org/wp-content/uploads/2019/10/stoichiometry-worksheet-answer-key-elegant-solution-stoichiometry-worksheet-of-stoichiometry-worksheet-answer-key.jpg
Ideal stoichiometry How many moles of ZnCl A 2 will be produced from 23 0 g of Zn assuming CuCl A 2 is available in excess Stuck Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more Khan Academy is a nonprofit with the mission of providing a free world class Posted on March 26 2015 by misterguch Stoichiometry sheets Stoichiometry I dd ch I love the smell of stoichiometry in the morning Stoichiometry Practice Worksheet The most fun you can have with a calculator More Exciting Stoichiometry Problems More fun for the whole chemist family
Chemistry library 20 units 54 skills Unit 1 Atoms compounds and ions Unit 2 More about atoms Unit 3 More about molecular composition Unit 4 Mass spectrometry Unit 5 Chemical reactions and stoichiometry Unit 6 More about chemical reactions Unit 7 Electronic structure of atoms Unit 8 Periodic table Stoichiometry Calculation Practice Worksheet Calculate the number of moles of NaOH that are needed to react with 500 g of H 2 SO 4 according to the following equation H 2 SO 4 2 NaOH Na 2 SO 4 2 H 2 O ANS 10 mol 2 Calculate the mass of NH 3 that can be produced from the reaction of 125 g of NCl 3 according to the following equation

Stoichiometry Worksheet 2 Answer Key HELLOINMYDREAM
https://www.compoundworksheets.com/wp-content/uploads/2023/03/stoichiometry-worksheet-2-answer-key-helloinmydream-791x1024.png

Stoichiometry Worksheet 2 Answers
https://i.pinimg.com/originals/46/04/a8/4604a8eb773a060ac9777f7367ea71fb.png
Stoichiometry 2 Worksheet Answers - Answer PROBLEM 5 2 1 5 5 2 1 5 Carborundum is silicon carbide SiC a very hard material used as an abrasive on sandpaper and in other applications It is prepared by the reaction of pure sand SiO 2 with carbon at high temperature Carbon monoxide CO is the other product of this reaction
