Rate Of Reaction Worksheet Answers 1 A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid The equation for the reaction is Zn s 2HCl aq o H2 g ZnCl2 aq A piece of zinc is dropped into 1 00 L of 0 100 M HCl and the following data were obtained Time Mass of Zinc 0 s 4 s 8 s 12 s 16 s 20 s 0 016 g
A Using the graph below calculate the rate of the reaction between the second and the fifth minute Rate slope 44mL 10mL 11 3 mL min When is the rate of the reaction the greatest Slope was steepest 3 4 min time interval When slope 0 rate 0 reaction is over 5 min Answer a Increases the speed of a Q2 A chemical reaction is of zero order when the reaction rate is where CA concentration of reactant a CA b 1 CA c Independent of temperature d None of these Answer d None of these Q3 The rate law for the reaction involved in inversion of cane sugar is R k C 12 H 22 O 11 H 2 O a True
Rate Of Reaction Worksheet Answers

Rate Of Reaction Worksheet Answers
https://d1e4pidl3fu268.cloudfront.net/4fa03714-12de-40ef-a121-bd7a5c4cde34/ResourcePreview.png

Chemistry Reaction Rates Worksheet Wildseasonthegame Db excel
https://db-excel.com/wp-content/uploads/2019/09/chemistry-reaction-rates-worksheet-wildseasonthegame-1.jpg

13 Best Images Of Worksheet Reaction Rates Answer Worksheet Measuring
http://www.worksheeto.com/postpic/2012/04/worksheet-measuring-reaction-rate_240180.png
This worksheet is designed to accompany chapter 14 in Chemistry Given the following energy diagram for a 3 step reaction answer the questions below Use your graph to determine the instantaneous reaction rate at 250 ms Given that the reaction is first order in NO and in O 3 determine the rate constant using your calculated Q4 Experimentally Determining the Rate Law Determine the rate law and value of k k for th e following reaction at 800 oC in a closed vessel Remember how concentration is related to pressure within the Ideal Gas law 2H2 g 2NO g 2H2O g N2 g 2 H 2 g 2 NO g 2 H 2 O g N 2 g Exp
To summarise the rate of reaction can be affected by 1 temperature 2 particle size and 3 concentration of the reactants Still another factor which can change a reaction s rate is a catalyst This is a substance which changes the rate of a reaction but remains itself un changed More support is available in this CPD article Teaching rates of reaction post 16 Another article Rationalising rates includes tips for teaching rates of reaction using graphs Prior knowledge In order for learners to succeed with rates of reaction at post 16 it is essential that they have a good knowledge
More picture related to Rate Of Reaction Worksheet Answers

Chemistry Rate Of Reaction Worksheet Cloze Passage Teaching Resources
https://d1e4pidl3fu268.cloudfront.net/755a63ad-56d1-40bc-9f21-d6815ac3cf33/Thumbnailsforrateofreactionworksheets.crop_720x540_0,7.preview.jpg

Rates Of Reaction Worksheet Db excel
https://db-excel.com/wp-content/uploads/2019/09/worksheet-11-calculating-reaction-rate-749x970.png

Quiz Worksheet Determining Rate Equation Rate Law Constant
https://study.com/academy/practice/quiz-worksheet-determining-rate-equation-rate-law-constant-reaction-order.jpg
1 Provide definitions for the following terms Catalyst a substance that causes or accelerates a chemical reaction without itself being affected Reaction Rate the speed at which a chemical reaction proceeds 2 Answer the following questions using COMPLETE SENTENCES a and b The mean rate of a chemical reaction will tell us how quickly or slowly that reaction takes place If a reaction has a low rate then it will happen slowly Reactions with a higher rate will happen much more quickly There are a number of factors that influence the rate from temperature to concentration
RATE OF REACTION WORKSHEET WITH ANSWER Subject Chemistry Age range 14 16 Resource type Worksheet Activity File previews docx 17 8 KB docx 1 8 MB The factors that affect the rate of chemical reactions are specifically dealt with in this worksheet plus some application questions involving temperature concentration surface area catalyst 2HCl aq CaCO3 s CaCl2 aq CO2 g H2O l If two experiments are carried out each using the same mass of CaCO3 marble chips and the same concentration of acid state two ways in which the second experiment could be changed to increase the rate of reaction 5 Define the term activation energy and explain how it affects the rate of a
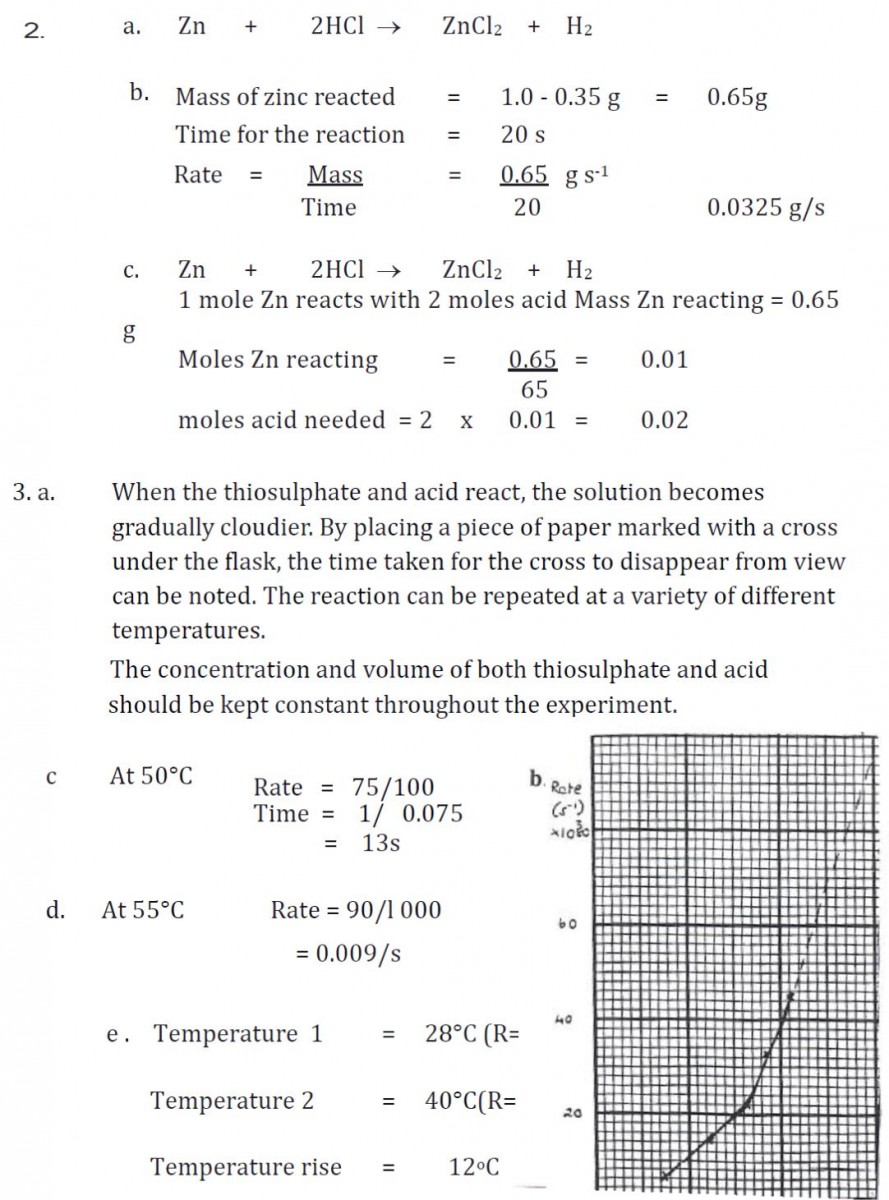
Answers Unit 1 Rates Of Reactions CfE Higher Chemistry Revision
https://blogs.glowscotland.org.uk/gc/public/hchemextra/uploads/sites/5401/2015/06/Picture11.jpg

Rate Of Reaction Practical Home Learning Worksheet GCSE Teaching
https://d1e4pidl3fu268.cloudfront.net/2f6dc88a-e5cd-46fa-bdb8-bdd5148770b7/C6RateofReactionPracticalPreview2.png
Rate Of Reaction Worksheet Answers - Q4 Experimentally Determining the Rate Law Determine the rate law and value of k k for th e following reaction at 800 oC in a closed vessel Remember how concentration is related to pressure within the Ideal Gas law 2H2 g 2NO g 2H2O g N2 g 2 H 2 g 2 NO g 2 H 2 O g N 2 g Exp