Properties Of Systems In Chemical Equilibrium Lab Answers Chemical equilibrium refers to a property of a system in which speed of forward and reverse reactions are the same By knowing what conditions are required to achieve desirable amount of product chemical equilibrium direction can be altered
Lab 2 Properties of Systems in Chemical Equilibrium The two key purposes of this lab are 1 To observe how systems in equilibrium respond to stress by increasing or decreasing the concentration of one component increasing the volume of a solution or changing the temperature of the system and Le Chatelier s principle describes how when a dynamic equilibrium is disrupted by a change of pressure temperature volume or concentration the position of equilibrium will shift to counteract any change and restore an equilibrium state
Properties Of Systems In Chemical Equilibrium Lab Answers

Properties Of Systems In Chemical Equilibrium Lab Answers
https://media.cheggcdn.com/media/4b9/4b9b38fe-ac4a-400d-8f18-0ed0bdc3ab5a/image.png
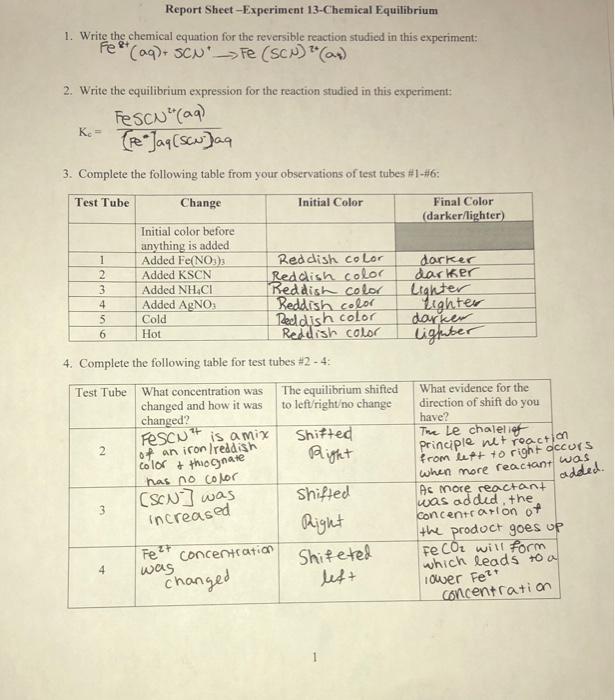
Solved Report Sheet Experiment 13 Chemical Equilibrium 1 Chegg
https://media.cheggcdn.com/study/864/86449366-022a-4686-b08a-79f082a51e1b/image
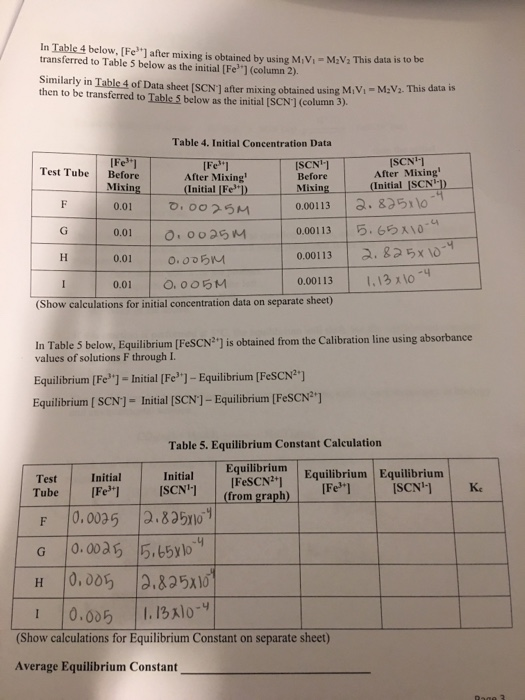
Determination Of An Equilibrium Constant Lab
https://media.cheggcdn.com/media/6cd/6cd905c0-0d60-4b34-8045-9e00a8b58ac8/image.png
In this experiment you will analyze two equilibrium systems The reaction for the first system is shown below and produces one colored complex ion Fe3 aq 6 SCN aq Fe SCN 6 3 aq Eq 1 The Fe SCN 6 3 complex ion forms a blood red solution We can induce a shift in this equilibrium and in so doing induce a color change in the Question PRE LAB EXERCISE Name Experiment 4 Properties of Systems in Chemical Equilibrium To be completed and turned in at the start of lalb Read through the procedure for todays experiment 1 Define Le Chatlier s Principle 2 How will Le Chatlier s Principle be used to a Cause a color change for an indicator in Part A b
A system at equilibrium is in a state of dynamic balance with forward and reverse reactions taking place at equal rates If an equilibrium system is subjected to a change in conditions that affects these reaction rates differently a stress then the rates are no longer equal and the system is not at equilibrium The system will subsequently experience a net reaction in the direction of Experiment 22 Properties of Systems in Chemical Equilibrium Le Ch telier s Principle Patricia Romero When working in the laboratory one often makes observations that at first sight are surprising and hard to explain One might add a reagent to a solution and obtain a precipitate
More picture related to Properties Of Systems In Chemical Equilibrium Lab Answers
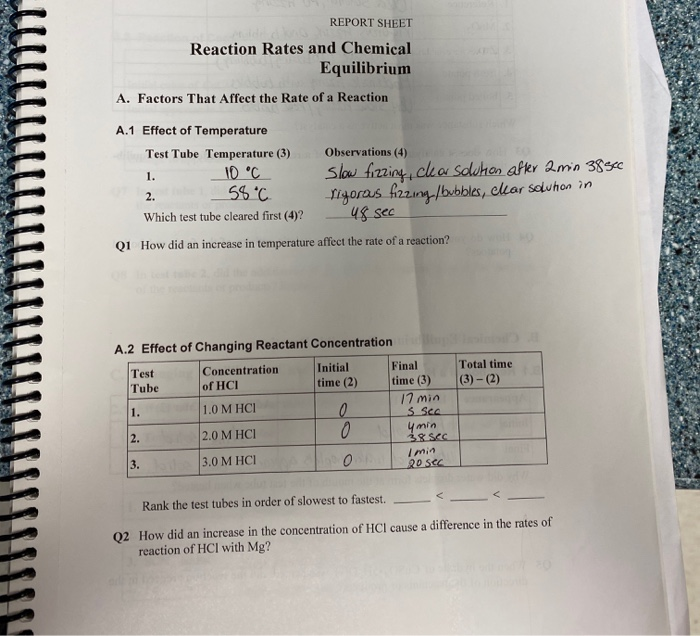
Solved REPORT SHEET Reaction Rates And Chemical Equilibrium Chegg
https://media.cheggcdn.com/study/dd9/dd9647b2-5db9-4289-91c2-7e6c01f77008/image.png

A Study Of Chemical Equilibrium Lab 36 Answers Study Poster
https://media.cheggcdn.com/media/ef7/ef7ef801-7428-4599-9efb-3640de22dca3/image.png

DOC Experiment 22 Properties Of Systems In Chemical Equilibrium Le
https://0.academia-photos.com/attachment_thumbnails/42682711/mini_magick20180817-21041-fmt0q5.png?1534564595
3 Interpret the measurable effects of disturbances to a system at equilibrium in terms of Le Ch telier s Principle PRE LAB Complete the Pre Lab Assignment at the end of this document before going to lab You will need some of the answers to these questions in order to get started with the experiment INTRODUCTION Question Experiment 10 Properties of Systems in Chemical Equilibrium Le Ch telier s Principle working in the laboratory one often makes observations that at first sight ure surpeising and hard to explain One might add a reagent to a solution and obtain a precipitate Addition of more of that reagent to the precipitate causes it to dissolve
Experiment 22 Properties of Systems in Chemical Equilibrium Data and Calculations Course Chemistry CHE 104 13 Documents CHE 104 post lab reflection Experiment 7 Chemistry None 2 CHE 104 post lab reflection Experiment 30 Chemistry None 3 Experiment 35 Spot Tests for Some Common Anions Chemistry Chemistry questions and answers Properties of Systems in Chemical Equilibrium Le Ch telier s Principle hen working in the laboratory one often makes observations that at first sight are surprising and hard to explain One might add a reagent to a solution and obtain a precipitate
Solved 184 Experiment 22 Properties Of Systems In Chemical Chegg
https://media.cheggcdn.com/media/dd1/dd1daa3a-cf4b-4a59-8065-2f5e04049dc4/image

Properties Of Systems In Chemical Equilibrium Le Chegg
https://media.cheggcdn.com/media/2c4/2c4d1823-dbb3-46af-9cc4-b00ba8d83f8b/image.png
Properties Of Systems In Chemical Equilibrium Lab Answers - In this experiment you will analyze two equilibrium systems The reaction for the first system is shown below and produces one colored complex ion Fe3 aq 6 SCN aq Fe SCN 6 3 aq Eq 1 The Fe SCN 6 3 complex ion forms a blood red solution We can induce a shift in this equilibrium and in so doing induce a color change in the