Mole Mole Stoichiometry Worksheet Answers A How many moles of O2 can be produced by letting 12 00 moles of KClO3 react 18 0 mol O2 3 Given the following equation 2 K Cl2 2 KCl a How many grams of KCl is produced from 2 50 g of K and excess Cl2 4 77 g KCl b How many grams of KCl is produced from 1 00 g of Cl2 and excess K 2 10 g KCl 4
7 Worksheets in Moles Stoichiometry Mole Conversions Practice converting moles Stoichiometry Mole Ratio Chemical reactions give information about the amount of MOLES involved the reaction The coefficients are the relative amounts of moles of each reactant and product used or produced in the reaction Worksheets General Chemistry Worksheets General Chemistry Guided Inquiry
Mole Mole Stoichiometry Worksheet Answers
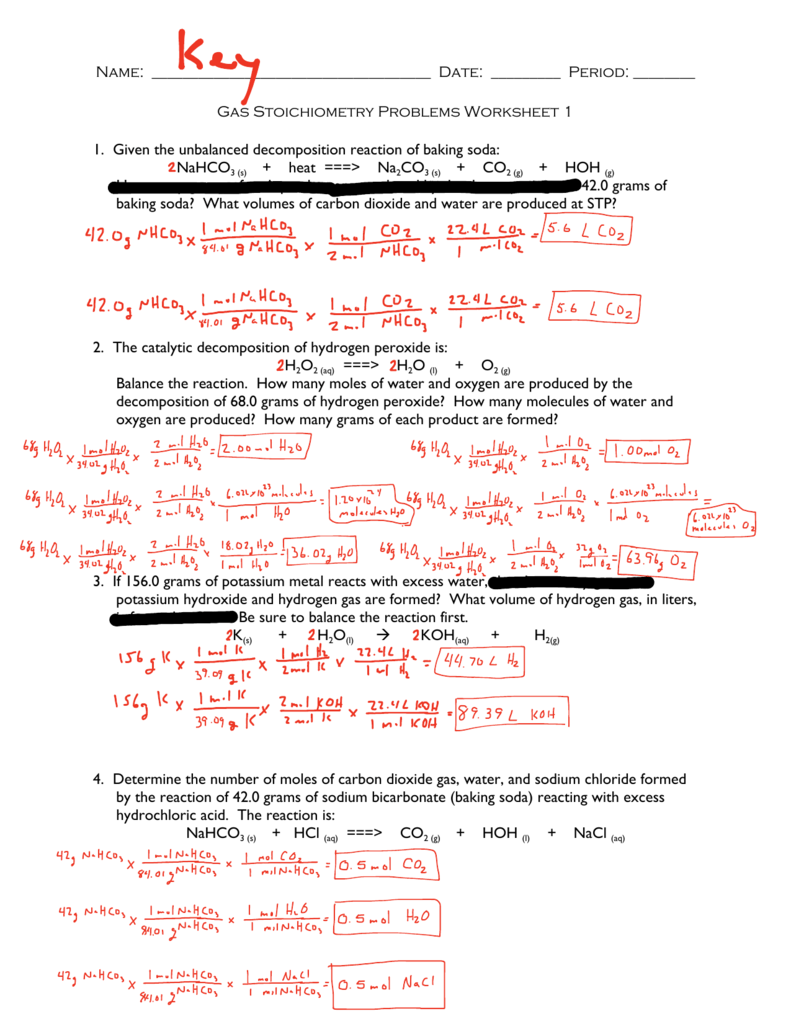
Mole Mole Stoichiometry Worksheet Answers
https://s3.studylib.net/store/data/008351424_1-9324fb6e101b050c986aab55e4ce5412.png

Mole Mole Stoichiometry Worksheet Answers
https://i.pinimg.com/originals/99/5e/6f/995e6fac9b0a0ebf5a558957a13a4a26.jpg

Stoichiometry Worksheet Mole Mole
https://i2.wp.com/www.worksheeto.com/postpic/2010/10/stoichiometry-practice-worksheet-answer-key_224021.jpg
1 The chemical equation of interest is this O CuSO 2 Every one mole of CuSO 4 5H 2 O that is heated releases five moles of water The ratio from the chemical equation is this 3 The ratio from the problem data is this 4 Solving x 8 75 moles of water will be produced 1 Based on the following equation how many moles of each product are produced when 5 9 moles of Zn OH 2 are reacted with H3PO4 You need to balance the equation Zn OH 2 H3PO4 Zn3 PO4 2 H2O 2 hydrogen are produced when 5 9 moles of aluminum reacts with excess hydrochloric acid
How is a mole like a dozen A dozen is twelve things and a mole is an Avogadro s number 6 022 x 1023 of things The difference is that a dozen things 12 is an exact number but a mole of things 6 022 x 1023 is an inexact measured number 4 Consider a 15 00 g sample of CO2 m w 44 01u How many moles of CO2 are there in this sample Q4 Given the following reaction H2SO4 Na2CO3 Na2SO4 H2O CO2 Calculate the molarity of the H2SO4 solution if it takes 40 0 mL of H2SO4 to neutralize 46 7 mL of a 0 364 M Na2CO3 solution
More picture related to Mole Mole Stoichiometry Worksheet Answers

16 Cool Stoichiometry Worksheet Mole To Mole
https://content.lessonplanet.com/resources/thumbnails/276135/original/odu4nda0lmpwzw.jpg?1414473411
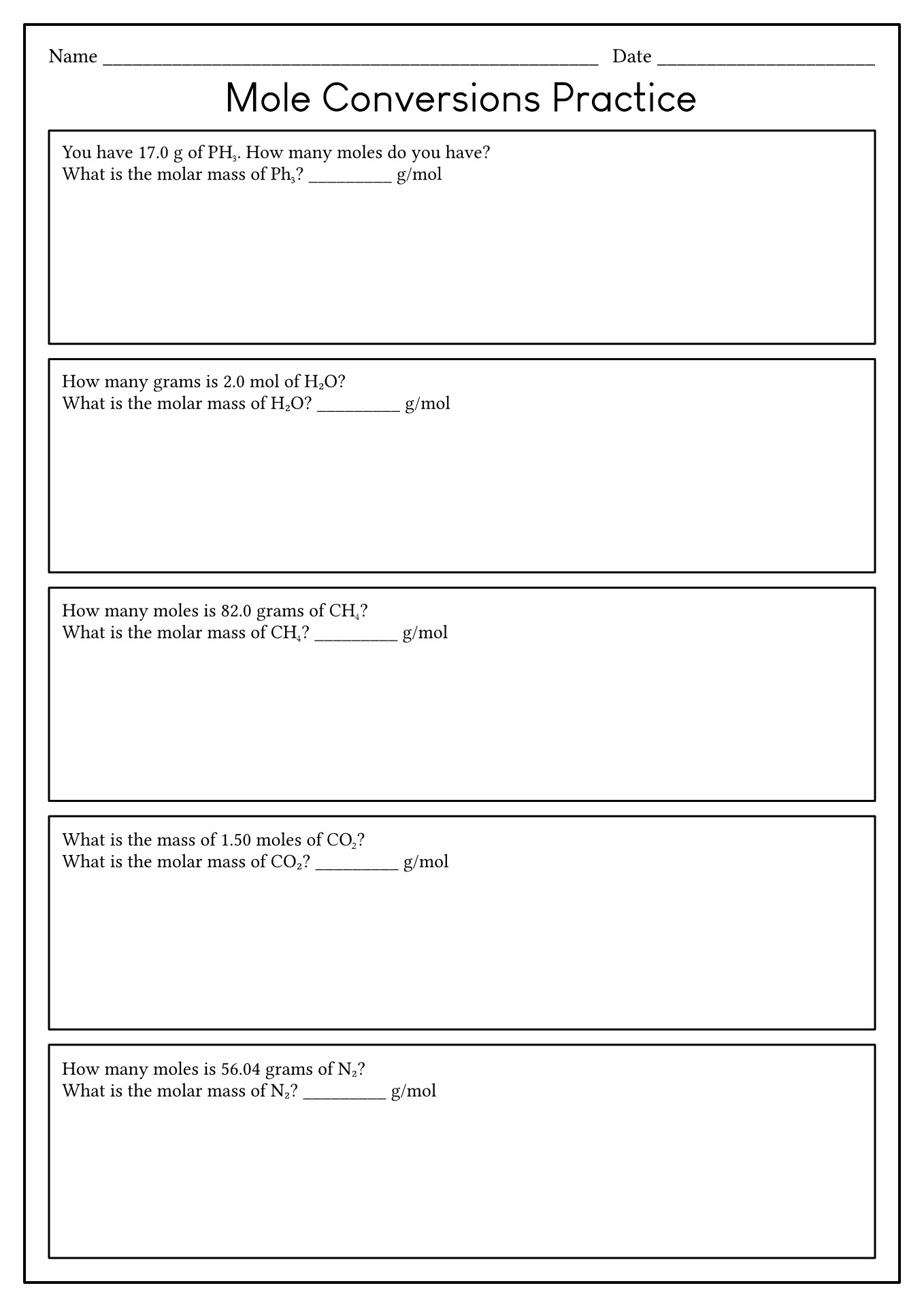
18 Mole Conversion Problems Worksheet Answers Free PDF At Worksheeto
https://www.worksheeto.com/postpic/2010/07/mole-conversion-worksheet_208026.png

Mole Mole Stoichiometry Worksheet Answers
https://s3.studylib.net/store/data/007734847_2-ccaf4ade60b8a5a6d3eb03b9a1dd62e3.png
Answers 1 40 08 grams of calcium is one mole see Periodic Table and one mole is 6 02 x 1023 atoms 2 One mole of anything is 6 02 x 1023 so it is 6 02 x 1023 Mg atoms 6 02 1023 3 5 55 mol Ag 3 34 x 1024 atoms Ag 1 4 1 5 55 x 1033 molecules 6 02 1023 9 22 x 109 moles H2SO4 5 Practice stoichiometry conversions with this bundle of worksheets Use this resource as classwork homework extra practice or examples with work shown for students in a distance learning setting A detailed answer key is included PLEASE NOTE All problems involving molar volume are considered to 5
A If you have 5 50 mol of CaC2 how much C2H2 do you get 143g C2H2 b How many moles of water are needed when 65 0g of CaC2 have reacted 2 03 mol H2O 4 In photosynthesis water reacts with carbon dioxide to give oxygen and glucose C6H12O6 Write and balance the chemical equation Mole Mole Problems Worksheet Mole Mass Problems Worksheet Mole Mole and Mole Mass Problems Mixed Problems Mole Mole and Mole Mass Worksheet Challenge Problem Stoichiometry This semester begins with the introduction of the mole

Stoichiometry Worksheet Answer Key
http://www.worksheeto.com/postpic/2015/10/mole-stoichiometry-worksheet-answers_224038.png

Mole Worksheet With Answers
http://www.worksheeto.com/postpic/2015/07/mole-ratio-worksheet-answers_224142.png
Mole Mole Stoichiometry Worksheet Answers - How is a mole like a dozen A dozen is twelve things and a mole is an Avogadro s number 6 022 x 1023 of things The difference is that a dozen things 12 is an exact number but a mole of things 6 022 x 1023 is an inexact measured number 4 Consider a 15 00 g sample of CO2 m w 44 01u How many moles of CO2 are there in this sample