Mass To Mass Stoichiometry Worksheet Example 12 4 1 12 4 1 Mass Mass Stoichiometry Ammonium nitrate decomposes to dinitrogen monoxide and water according to the following equation NH4NO3 s N2O g 2H2O l NH 4 NO 3 s N 2 O g 2 H 2 O l In a certain experiment 45 7g 45 7 g of ammonium nitrate is decomposed Find the mass of each of the products formed
This answer may then be converted to grams of SO 3 10 7 7 mol SO3 80 07gSO3 1 molSO3 862gSO3 10 7 7 m o l S O 3 80 07 g S O 3 1 m o l S O 3 862 g S O 3 The final answer is expressed to three significant figures Thus in a two step process we find that 862 g of SO 3 will react with 3 59 mol of Fe 2 O 3 Mass to Mass or Mass to Mole Conversions Objective Given the mass one species be able to predict the mass another species consumed or produced from a balanced chemical equation Technique This is a three step process which should be done in one equation which uses three conversion factors Conversion Factor 1 Use molar mass to convert mass
Mass To Mass Stoichiometry Worksheet
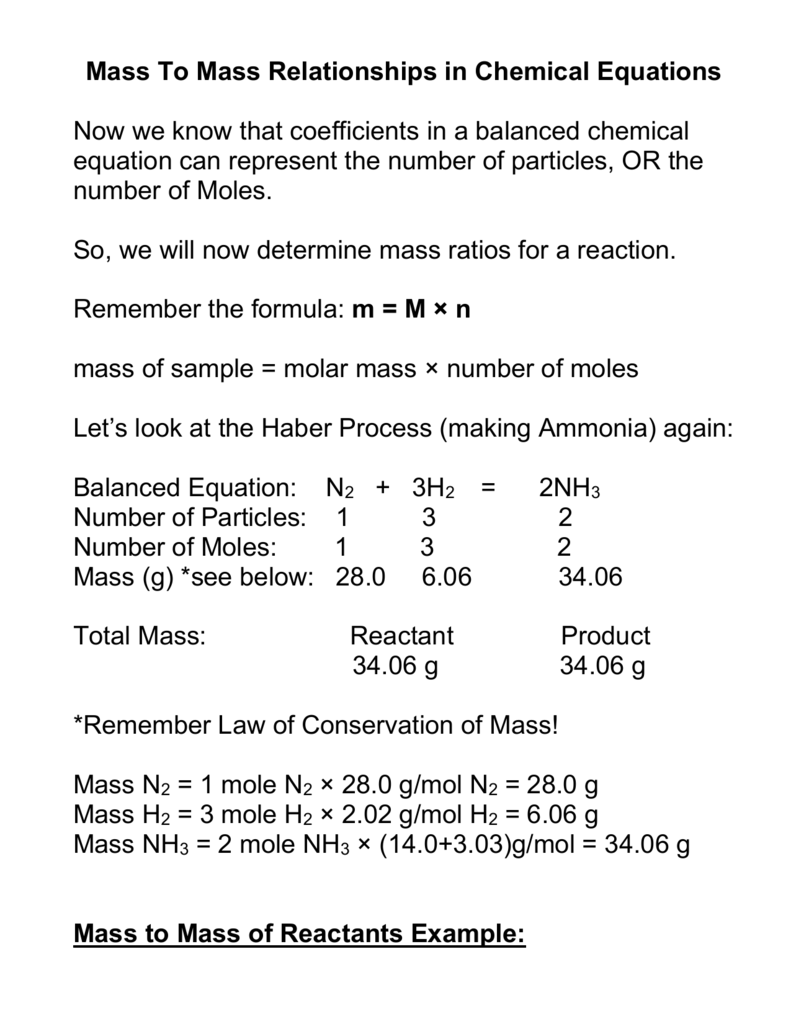
Mass To Mass Stoichiometry Worksheet
https://s3.studylib.net/store/data/009037666_1-90282412b2cc0b631a5e5bb3b9eb3c6c.png
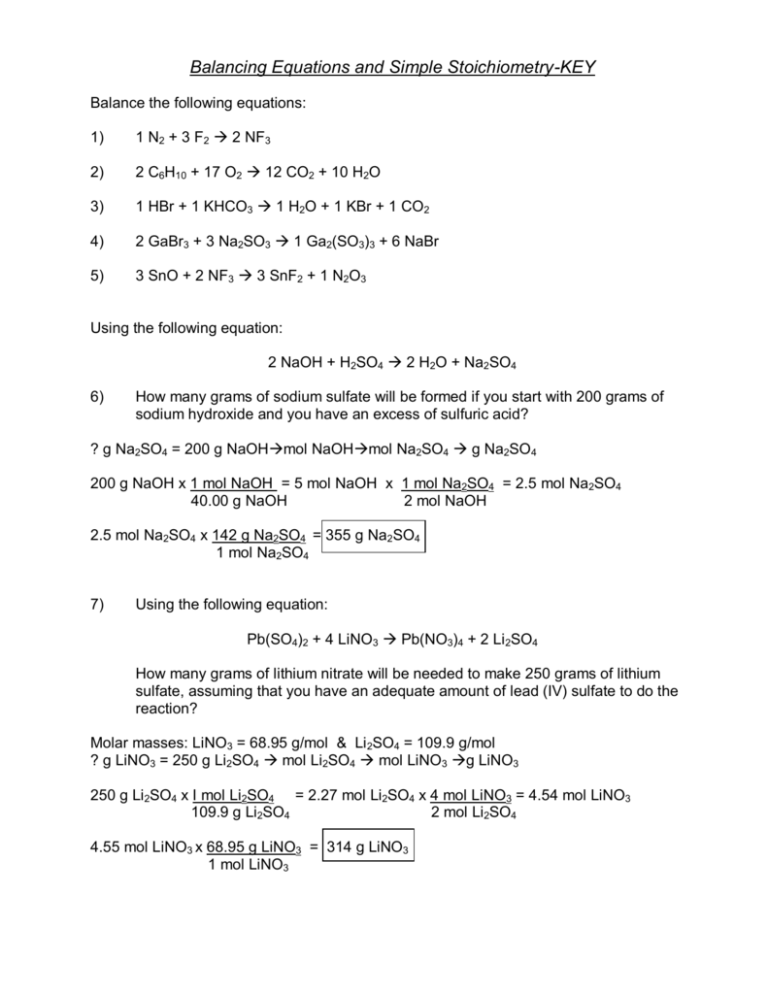
KEY Solutions For The Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008711874_1-d12f29b6b62688faeae95fc3bd7c9543-768x994.png

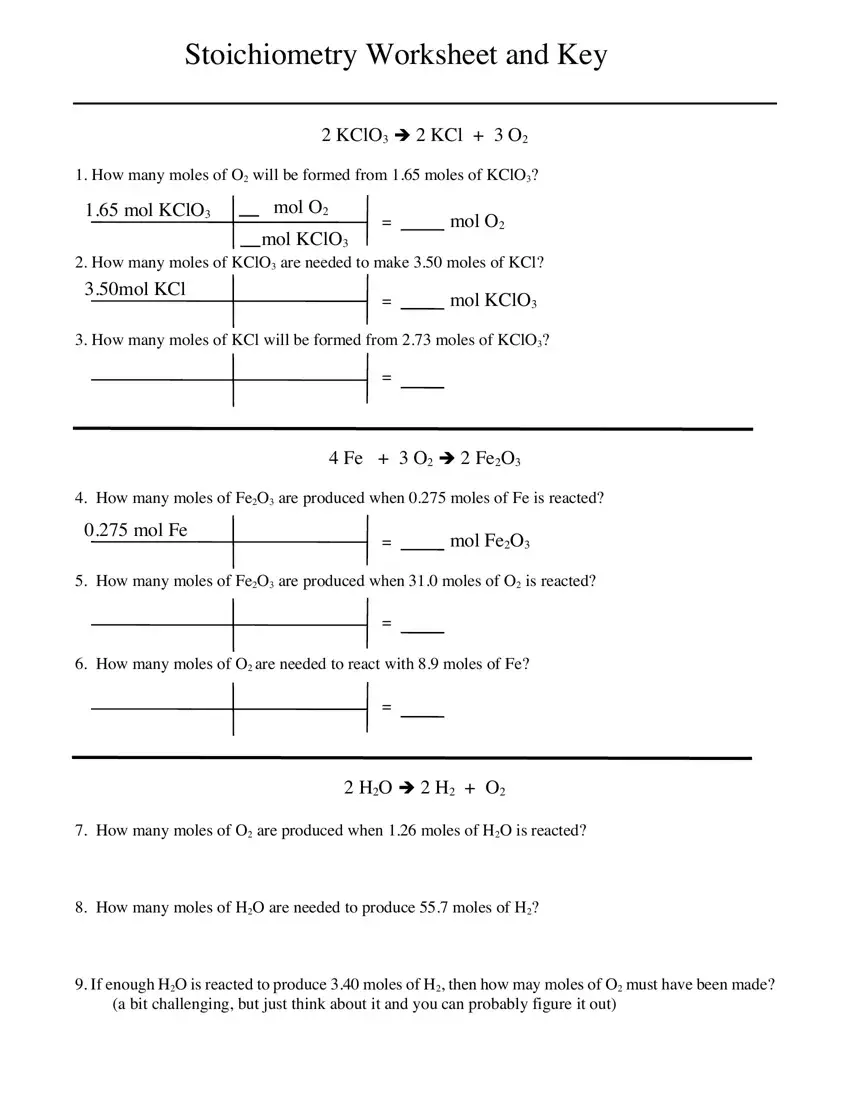
Stoichiometry Practice Worksheet
https://s3.studylib.net/store/data/008548006_1-87998e5b1b015fdc34a74176a67dd4ab.png
Mass Mass Examples Problems solved using dimensional analysis only Return to Stoichiometry Menu This is the most common type of stoichiometric problem in high school There are four steps involved in solving these problems Make sure you are working with a properly balanced chemical equation Apply mass to mass stoichiometry Google Classroom You might need Calculator Periodic table Table salt or sodium chloride can be decomposed into sodium metal and chlorine gas by running electricity through molten NaCl A Downs cell produces sodium and chlorine from molten sodium chloride The balanced equation for this reaction is
The equation for the reaction is 2NaCl l 2Na l Cl2 g Calculate the mass of chlorine gas that would be produced from 5 00 102 mol of molten sodium chloride 4 Copper metal reacts with 7 mol dm 3 nitric acid to produce nitrogen monoxide among other products The mass of each mole of hydrogen atoms is 1 008 g so multiply this by two to get 2 016 g The mass of one mole of oxygen atoms is 15 999 2 016 15 999 18 015 g mol This can be used to
More picture related to Mass To Mass Stoichiometry Worksheet

Gram To Gram Stoichiometry Worksheet Answers
https://i.pinimg.com/736x/bb/30/b0/bb30b04ec67c68ac872087205bebf8f5.jpg
Stoichiometry Worksheet Fill Out Printable PDF Forms Online
https://formspal.com/pdf-forms/other/stoichiometry-worksheet/stoichiometry-worksheet-preview.webp

SOLUTION Stoichiometry Worksheet Pt 1 Studypool
https://sp-uploads.s3.amazonaws.com/uploads/services/2387056/20211030121328_617d36e84b426_stoichiometry_worksheet_pt.1page2.png
Instructions Before viewing an episode download and print the note taking guides worksheets and lab data sheets for that episode keeping the printed sheets in order by page number During the lesson watch and listen for instructions to take notes pause the video complete an assignment and record lab data A If you have 5 50 mol of CaC2 how much C2H2 do you get 143g C2H2 b How many moles of water are needed when 65 0g of CaC2 have reacted 2 03 mol H2O 4 In photosynthesis water reacts with carbon dioxide to give oxygen and glucose C6H12O6 Write and balance the chemical equation
The mass of product depends upon the mass of limiting reactant The stoichiometry close stoichiometry The ratio of the amounts of each substance in a balanced chemical equation of a reaction A How many moles of dinitrogen tetrahydride are required to produce 57 moles of nitrogen B How many moles of dinitrogen tetroxide are required to produce 57 moles of nitrogen C How many moles of water are produced when 57 moles of nitrogen are made 3 Calculate the mass of aluminum oxide produced when 3 75 moles of aluminum burn in oxygen

Stoichiometry Worksheet 10 Problems With Mass To Mass Calculations
https://media.madebyteachers.com/wp-content/uploads/2023/01/25053848/mtms-thumb-500x500.png
Solved Worksheet For Basic Stoichiometry Part 1 Mole A Mass Chegg
https://media.cheggcdn.com/media/f6b/f6b990df-cc62-4524-988f-341d7f51f886/php3mB8qv
Mass To Mass Stoichiometry Worksheet - The equation for the reaction is 2NaCl l 2Na l Cl2 g Calculate the mass of chlorine gas that would be produced from 5 00 102 mol of molten sodium chloride 4 Copper metal reacts with 7 mol dm 3 nitric acid to produce nitrogen monoxide among other products
