M M Balancing Equations Lab Answers 42 4 5K views 6 years ago 8th grade Middle School Students at Rogers Herr Middle School may have needed a little bit more help understanding how to balance chemical equations when we used M Ms
M M s Balancing Equations Lab Background The law of conservation of mass states Your Goal To use M Ms to model a chemical equation and then balance them Directions DO NOT EAT THEM 2 Set up the reactants using the correct number of M M s Move the same M M s to the product side Analysis Questions 1 What do the M M s stand for in this lab 2 What is the coefficient and what does it apply to 3 What are the steps to balancing a chemical equation 4 How does the Law of Conservation of Matter apply to chemical reactions Balance these equations 1 Zn HCl ZnCl H2 2 KNO3 KNO2 O2 3
M M Balancing Equations Lab Answers

M M Balancing Equations Lab Answers
https://i2.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-11.jpg

Chemistry 101 Balancing Equations Worksheet Answers Tessshebaylo
https://media.cheggcdn.com/media/f12/f12f9179-c8c6-41fb-865c-c5263cb042de/image.png

Balancing Chemical Equations Using Skittles The Kiddos Love Getting To
https://i.pinimg.com/originals/e9/9f/ea/e99feaea6c5f6de184999d1a1c7bf465.jpg
Upload 3 pictures that show the use of the modeling kit Each picture should balance one of the equations bellow with the modeling kit Laboratory Questions 10 point total 1 point each Balancing Chemical Equations Balance the following equations using the model pieces and Balancing Equations mat HCl NaOH NaCl H 2 O 1 Use the M M s to model atoms 2 Set up the reactants using the correct number of M M s Move the same M M s to the product side As they become more difficult you may need to set up the products first and work backwards 3 Set of the molecules and fill in the coefficients 4 Draw and color the equations that represent the reactions
Try to balance the equation first I will check and initial these before you move on to model the equation using beads b String the beads onto the yarn in order to represent molecules in which all the atoms are attached together You may tie these LOOSLY if it helps but you will need to use them again so do not tie the string too tight About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright
More picture related to M M Balancing Equations Lab Answers

Balancing Equations Lab Instructional Video YouTube
https://i.ytimg.com/vi/i9VoLQPMrIA/maxresdefault.jpg

Exemplary 100 Examples Of Chemical Equations Balanced Molecular
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-43.jpg

Phet Balancing Equations Answer Key Phet Balancing Equations
https://i.ytimg.com/vi/GneOr6091qU/maxresdefault.jpg
Google Classroom Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint Report a problem Do 4 problems Answer to Solved Balancing Chemical Equations Lab 7 Balancing Chegg
Step 1 Given The reactions given are Ni NO A 3 A 2 aq 2 NaOH aq Ni OH A 2 s 2 NaNO A 3 aq View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text 1 Use the MM s to model atoms 2 Set up the reactants using the correct number of MM s Move the same MM s to the product side As they become more difficult you may need to set up the products first and work backwards 3 Set of the molecules and fill in the coefficients 4 Draw and color the equations that represent the
49 Balancing Chemical Equations Worksheets with Answers Worksheet
https://lh5.googleusercontent.com/proxy/R_rfkBkGT8IFYDIsuni9wzwCAubKpe9sTDAGTh01rRv_RvM5xyJj6z9OZd7rt3pUOLFoSntZ2FkGfBEj1wlYCI2ajYDWJ_KoESYu0lRVS3vj5fbEZBS5xsao3SYrFLGfuHncQaRGD0XbGG6xzUfm6ccDs6a9XbE77N6seA=s0-d
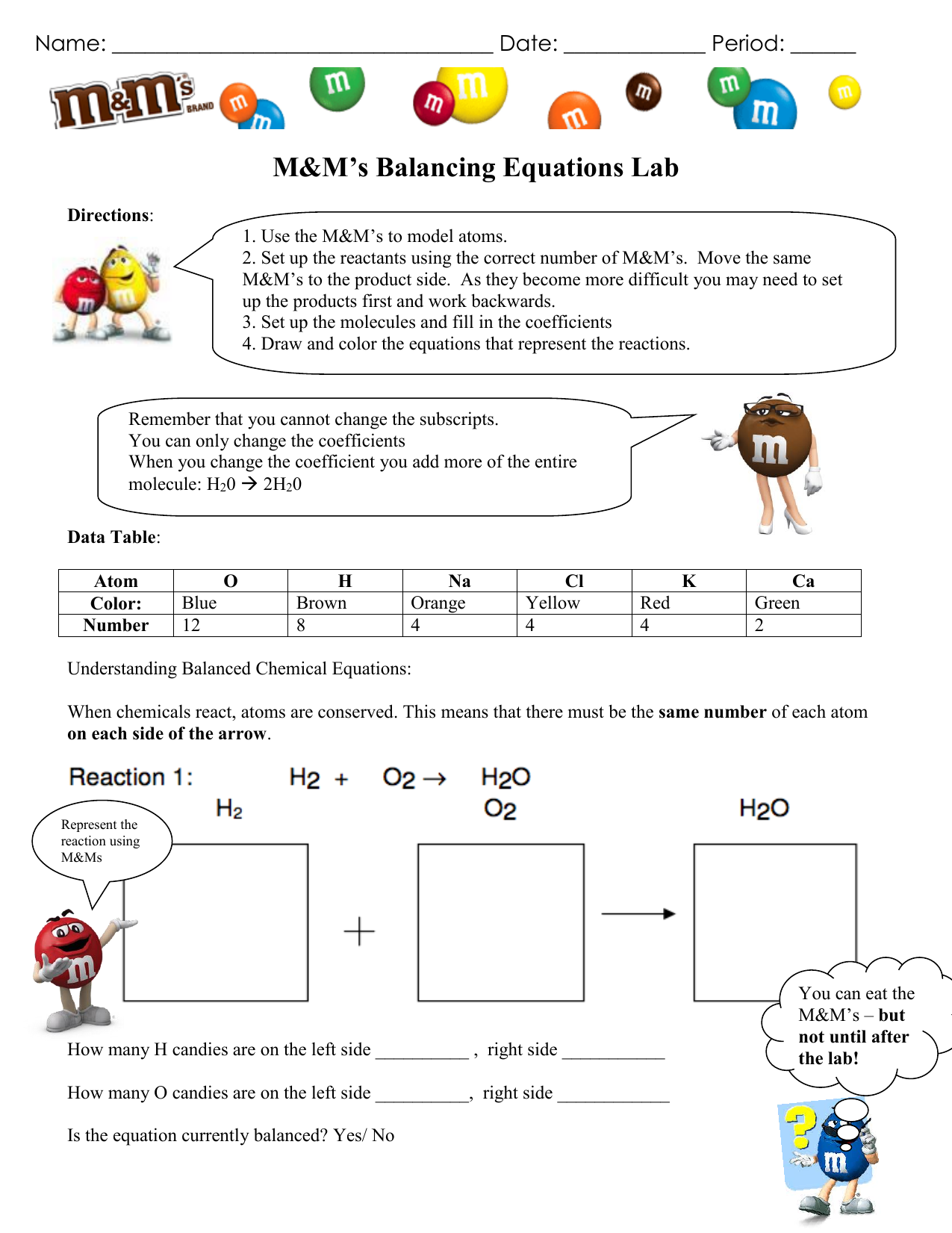
M M Balancing Equations Answer Key Balancing Equations Lesson Plan
https://s3.studylib.net/store/data/025287813_1-bccf1b2ad3b817f48f14436ba645da34.png
M M Balancing Equations Lab Answers - 1 Use the M M s to model atoms 2 Set up the reactants using the correct number of M M s Move the same M M s to the product side As they become more difficult you may need to set up the products first and work backwards 3 Set of the molecules and fill in the coefficients 4 Draw and color the equations that represent the reactions