M M Balancing Equations Lab Answer Key 42 4 5K views 6 years ago 8th grade Middle School Students at Rogers Herr Middle School may have needed a little bit more help understanding how to balance chemical equations when we used M Ms
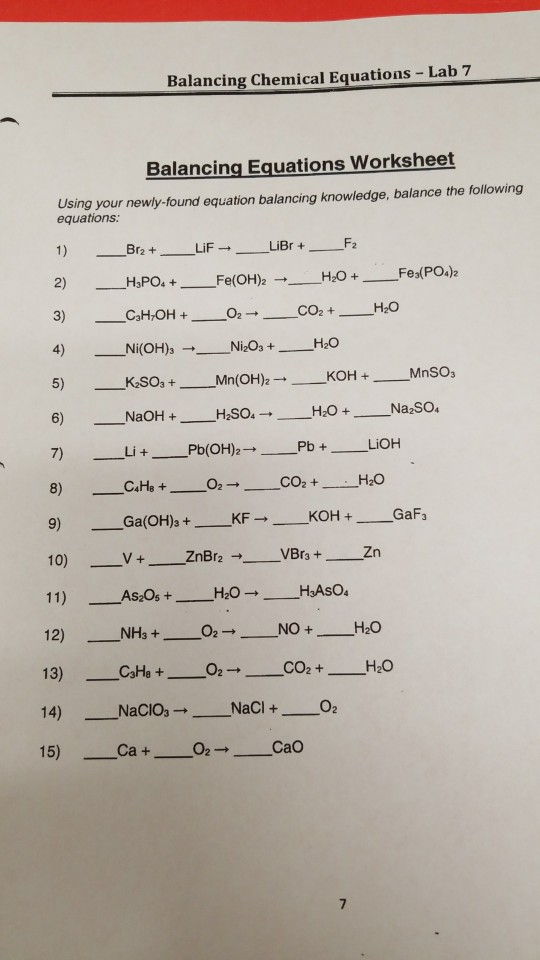
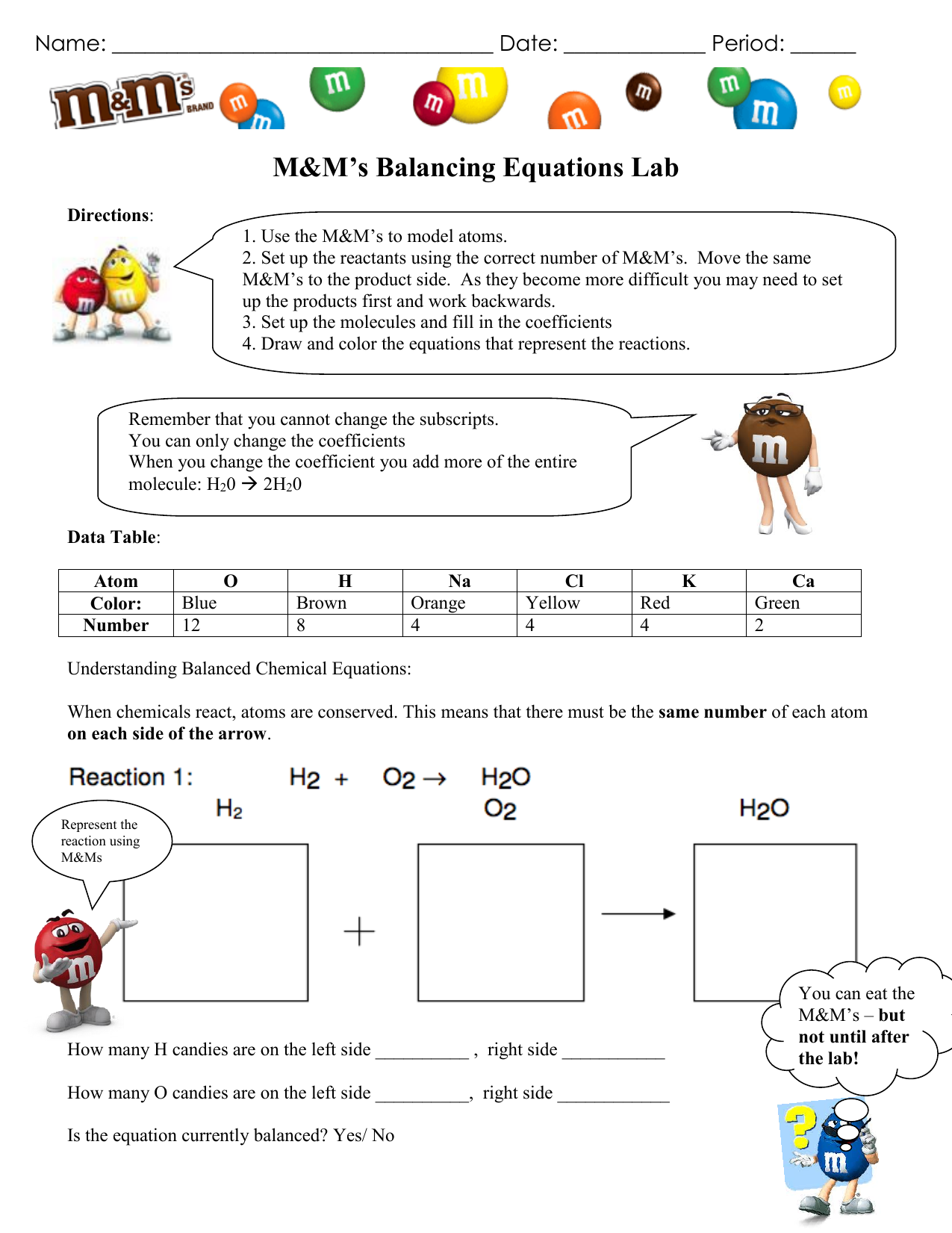
M M s Balancing Equations Lab Directions 1 Use the M M s to model atoms 2 Set up the reactants using the correct number of M M s Move the same M M s to the product side As they become more difficult you may need to set up the products first and work backwards 3 M M s Balancing Equations Lab Background The law of conservation of mass states Your Goal To use M Ms to model a chemical equation and then balance them Directions DO NOT EAT THEM 2 Set up the reactants using the correct number of M M s Move the same M M s to the product side
M M Balancing Equations Lab Answer Key

M M Balancing Equations Lab Answer Key
http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-07.jpg?is-pending-load=1
Balancing Chemical Equations Phet Lab Answer Key Tessshebaylo
https://s3.studylib.net/store/data/025495766_1-8d6c8ac5f733a77ed65c14dfca13721f-768x994.png
Answer Key Balancing Equations Worksheet Displaying 8 Worksheets For
https://d2vlcm61l7u1fs.cloudfront.net/media/ece/ece8d0e0-7d2e-41d8-9d13-4c1b348fa230/image
1 Use the M M s to model atoms 2 Set up the reactants using the correct number of M M s Move the same M M s to the product side As they become more difficult you may need to set up the products first and work backwards 3 Set of the molecules and fill in the coefficients 4 Draw and color the equations that represent the reactions About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright
PROCEDURE I Obtain a petri dish of colored beads see table below for quantity of each If your dish of beads does not have enough get them from the reserve stockpile at the teacher s desk The numbers shown below are the minimum for you to be able to do the equation balancing II For equations 1 5 below complete the following steps Analysis Questions 1 What do the M M s stand for in this lab 2 What is the coefficient and what does it apply to 3 What are the steps to balancing a chemical equation 4 How does the Law of Conservation of Matter apply to chemical reactions Balance these equations 1 Zn HCl ZnCl H2 2 KNO3 KNO2 O2 3
More picture related to M M Balancing Equations Lab Answer Key

Balancing Equations Worksheet 1 Answer Key Equations Worksheets
https://i0.wp.com/www.unmisravle.com/wp-content/uploads/2018/04/balancing_equations_practice_worksheet_answers_2.jpg
Worksheet More Practice Balancing Equations Balance The Following
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-15.jpg

M Ms Balancing Chemic YouTube
https://i.ytimg.com/vi/sA6oljQOnRA/maxresdefault.jpg
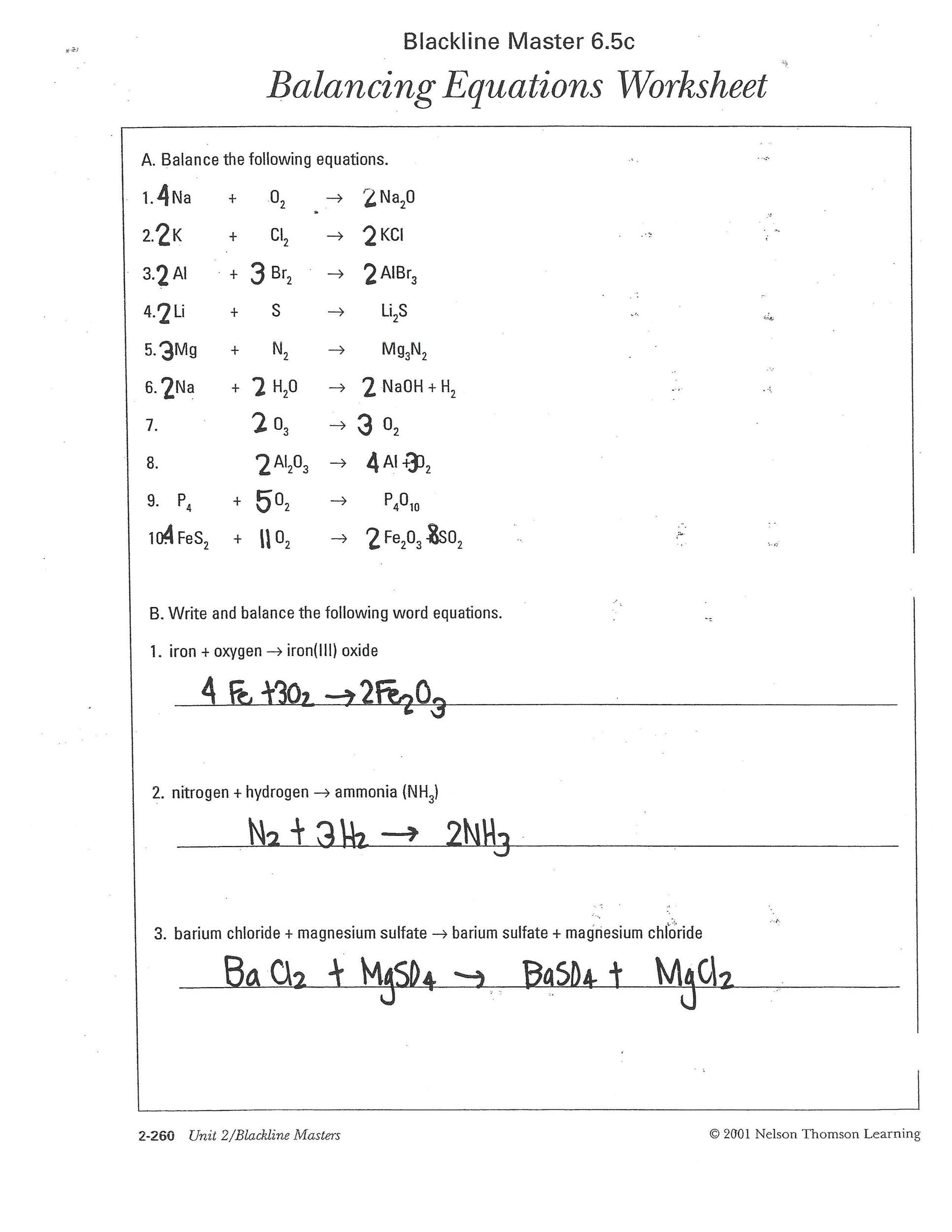
Balancing Chemical Equations Answer Key Balance the equations below 1 1 N2 3 H2 2 NH3 2 2 KClO3 2 KCl 3 O2 3 2 NaCl 1 F2 2 NaF 1 Cl2 4 2 H2 1 O2 2 H2O 5 1 Pb OH 2 2 HCl 2 H2O 1 PbCl2 6 2 AlBr3 3 K2SO4 6 KBr 1 Al2 SO4 3 7 1 CH4 2 O2 1 CO2 2 H2O 8 1 C3H8 5 O2 3 CO2 4 H2O 9 2 C8H18 Not quite what you were looking for Search by keyword to find the right resource Worksheet for balancing chemical equations Used as a starter after a lesson on balancing equations Pupils balance the equations using m m s they place the swe
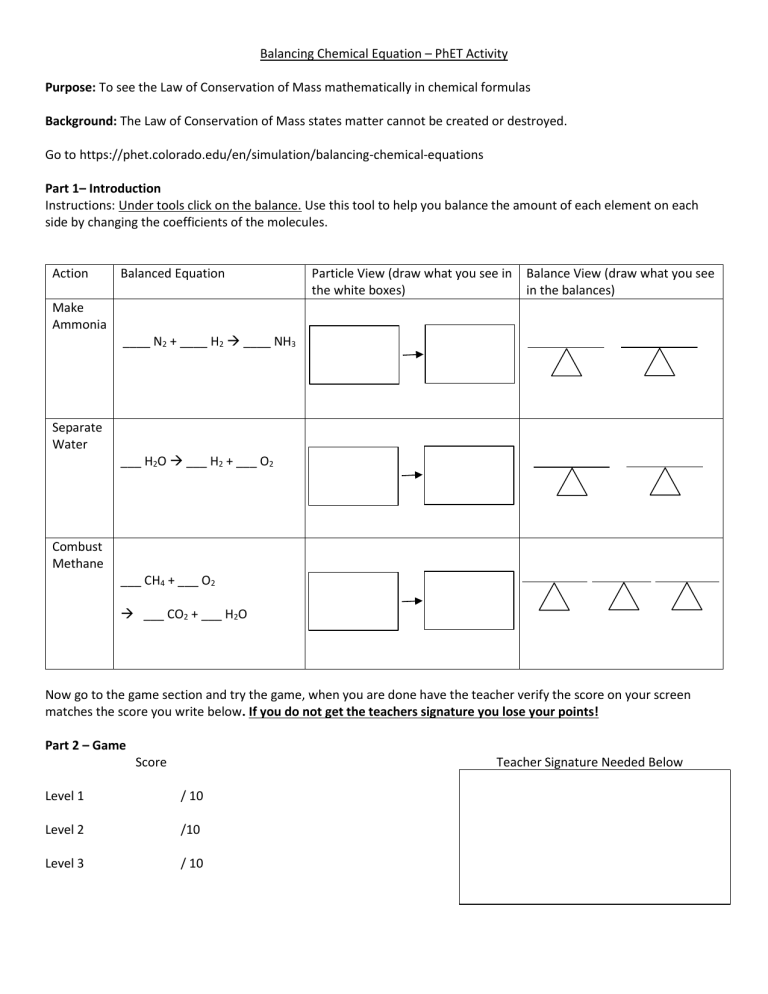
Learn how to balance chemical equations by using the law of conservation of mass and the coefficients of reactants and products Practice with different levels of difficulty and get immediate feedback Compare your results with real life examples and simulations of chemical reactions The Key to Balancing Chemical Equations The ultimate goal for balancing chemical equations is to make both sides of the reaction the reactants and the products equal in the number of atoms per element This stems from the universal law of the conservation of mass which states that matter can neither be created nor destroyed
M M Balancing Equations Answer Key Balancing Equations Lesson Plan
https://s3.studylib.net/store/data/025287813_1-bccf1b2ad3b817f48f14436ba645da34.png

49 Balancing Chemical Equations Worksheets with Answers
https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-17.jpg
M M Balancing Equations Lab Answer Key - Example 1 Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen N 2 and oxygen O 2 to form dinitrogen pentoxide reveal answer q 463373 Show Answer reveal answer hidden answer a 463373 First write the unbalanced equation Next count the number of each type of atom present in the