Identifying Reaction Types And Balancing Equations Answer Key Identify Type of Reaction Single Displacement Balance ions not atoms Video 3 3a Double and Single Displacement Balance ions not atoms Video 3 3b Strategy is pick an ion do its counterion then do the counterion s counterion and repeat until all have been balanced watch video Combustion
Balancing Simple Chemical Equations When a chemist encounters a new reaction it does not usually come with a label that shows the balanced chemical equation Instead the chemist must identify the reactants and products and then write them in the form of a chemical equation that may or may not be balanced as first written The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
Identifying Reaction Types And Balancing Equations Answer Key
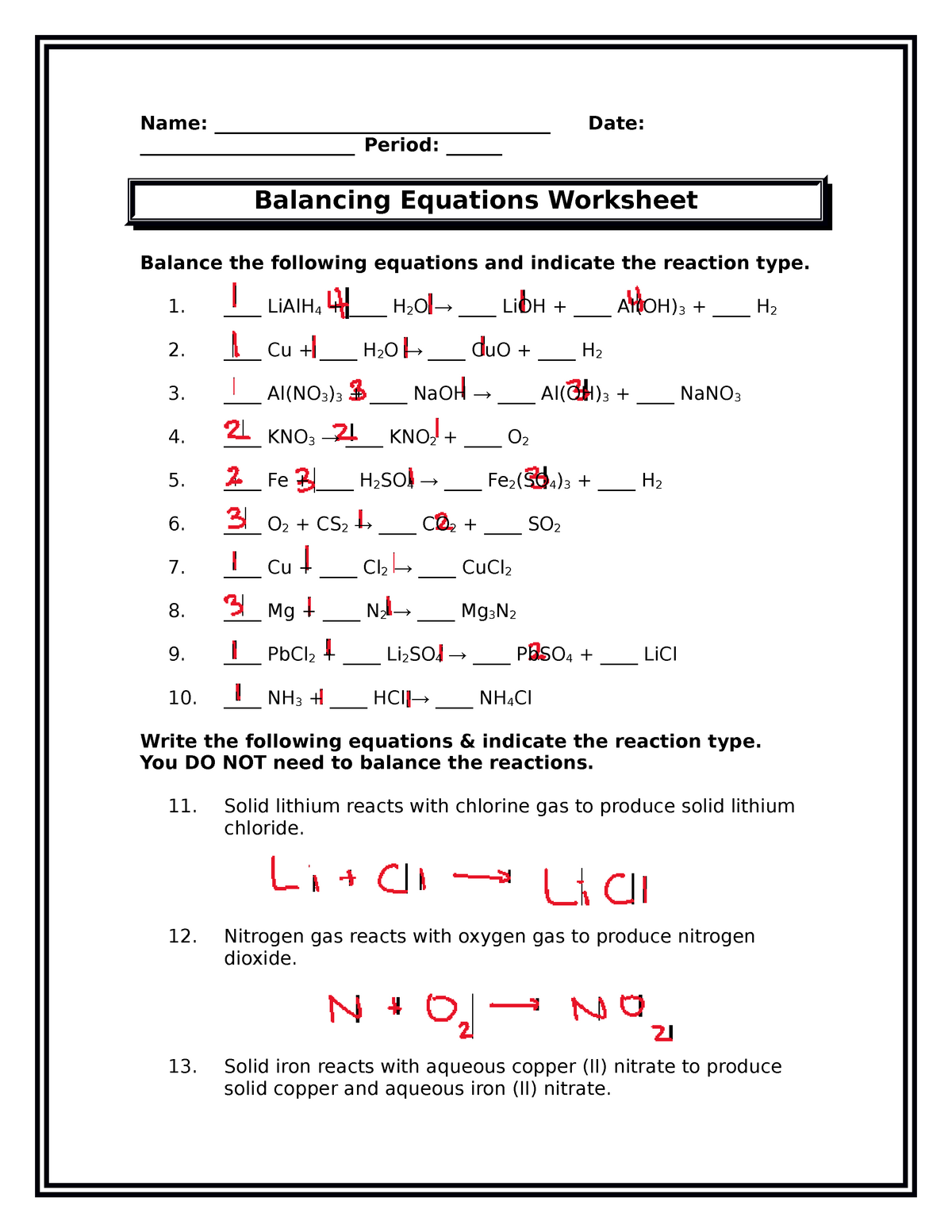
Identifying Reaction Types And Balancing Equations Answer Key
https://d20ohkaloyme4g.cloudfront.net/img/document_thumbnails/9c7b088249d348f4a3576549a900fb63/thumb_1200_1553.png

14 Best Images Of Types Of Reactions Worksheet Answers Balancing
http://www.worksheeto.com/postpic/2013/12/balancing-chemical-equations-worksheet-answer-key_243178.jpg

Balancing Equations And Types Of Reactions Worksheet Answers
https://i0.wp.com/db-excel.com/wp-content/uploads/2019/09/11-balancing-chemical-equations-1-2-k-1.png
Even though chemical compounds are broken up and new compounds are formed during a chemical reaction atoms in the reactants do not disappear nor do new atoms appear to form the products In chemical reactions atoms are never created or destroyed Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint
Types of Reactions Worksheet Solutions Balance the following equations and indicate the type of reaction taking place 1 3 NaBr 1 H3PO 4 1 Na 3PO 4 3 HBr Type of reaction double displacement 2 3 Ca OH 2 1 Al 2 SO 4 3 3 CaSO 4 2 Al OH 3 Type of reaction double displacement 3 3 Mg 1 Fe 2O3 2 Fe 3 MgO Type of reaction single displacement 4 1 C2H4 3 O2 2 CO 2 2 H2O N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have
More picture related to Identifying Reaction Types And Balancing Equations Answer Key

49 Balancing Chemical Equations Worksheets with Answers
http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-07.jpg?is-pending-load=1

Balancing Chemical Equations Worksheet For Class 8
https://i0.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-14.jpg

Balancing Chemical Equations Worksheet For Class 10
https://i0.wp.com/templatelab.com/wp-content/uploads/2017/01/balancing-equations-43.jpg
Sixteen balance redox equations by sight Single Replacement Double Replacement Decomposition Synthesis Combustion Writing molecular and net ionic equations 10 15 20 A list of the seven diatomic elements plus two friends Reaction types and product prediction in a PDF file Identify the type of reaction and balance the equation The number in boldface is the SUM of the coefficients of the correctly balanced equation For the following reactions indicate whether the following are examples of synthesis decomposition combustion single displacement double displacement or acid base reactions
Describe each type of chemical reaction Differentiate between the types of chemical reactions Understand how each reaction type correlates to real life Give examples of each type of reaction This document is intended to help you review the basics of writing and balancing equations how to predict the products of several general types of inorganic reactions and how to write and balance equations for the combustion of hydrocarbons There are also practice exercises with answers

Balancing Chemical Equation Worksheet 1
http://templatelab.com/wp-content/uploads/2017/01/balancing-equations-36.jpg

Dlewis Blog
http://3.bp.blogspot.com/-i26EUcUftQo/T5lTCqp5xuI/AAAAAAAAA7I/Ec5FzeRYNfw/s1600/Untitled_1.jpeg
Identifying Reaction Types And Balancing Equations Answer Key - N2 H2 NH3 On the left there is 2 N and 2 H On the right there is 1 N and 3 H If we tried to balance starting with H you d need to use a fraction or decimal and would get messy so let s start with N There s 2 on the left and 1 on the right so we need to change the coefficient of NH3 to 2 Now we have