How Many Moles Of N2 And H2 Were Present Originally 1 29 minutes Problem 103 Brown 14th Edition Textbook Question A mixture of N21g2 and H21g2 reacts in a closed container to form ammonia NH31g2 The reaction ceases before either reactant has been totally consumed At this stage 3 0 mol N2 3 0 mol H2 and 3 0 mol NH3 are present How many moles of N2 and H2 were present originally
So we re going to need 0 833 moles of molecular oxygen And then I just multiply that times the molar mass of molecular oxygen So times 32 00 grams per mole of molecular oxygen 0 833 times 32 is equal to that If you go three significant figures it s 26 7 26 7 grams of oxygen of molecular oxygen N2 g 3 H2 g 2 NH3 g H 92kJ mol Answer Increasing the temperature will cause the equilibrium to shift towards the reactants left This is because the reaction is exothermic and releases heat So if you add heat more N2 g and H2 g will be created and balance out the reaction
How Many Moles Of N2 And H2 Were Present Originally
How Many Moles Of N2 And H2 Were Present Originally
https://search-static.byjusweb.com/question-images/aakash_pdf/99996148753-0-0
Mole Ratio For N2 H2 NH3 YouTube
https://i.ytimg.com/vi/fn0Lnj2vuvA/maxresdefault.jpg
Q 2 Mole Of N2 Is Mixed With 6 Moles Of H2 In A Closed Vessel Of 1
https://search-static.byjusweb.com/question-images/aakash_pdf/99996245853-0-0
B Because the coefficients of gold and the Au CN 2 ion are the same in the balanced chemical equation assuming that Zn s is present in excess the number of moles of gold produced is the same as the number of moles of Au CN 2 i e 0 132 mol of Au The problem asks for the mass of gold that can be obtained so the number of We would speak of this equation as one mole of molecular phosphorus reacts with five moles of elemental oxygen to make one mole of tetraphosphorus decoxide Exercise 11 7 1 1 11 7 1 1 Interpret this balanced chemical equation in terms of moles N2 3H2 2NH3 N 2 3 H 2 2 NH 3 Answer
Are present How many moles of N 2 N 2 and H 2 H 2 were present originally Solution Verified Create a free account to view solutions Recommended textbook solutions Chemistry The Molecular Nature of Matter and Change 7th Edition ISBN 9780073511177 Patricia Amateis Silberberg 6 032 solutions Chemistry The Central Science Howmany moles of N2 and H2 were present originally A mixture of N2 g and H2 g reacts in a closed containerto form ammonia NH3 g The reaction ceases beforeeither reactant has been totally consumed At this stage3 0 mol N2 3 0 mol H2 and 3 0 mol NH3 are present Howmany moles of N2 and H2 were present originally BUY
More picture related to How Many Moles Of N2 And H2 Were Present Originally
When 1 Mole Of N2 And 1 Mole Of H2 Is Enclosed In 3 L Vessel And The
https://search-static.byjusweb.com/question-images/aakash_pdf/99996206606-0-0

Answered How Many Moles Of H2 Are Required To Bartleby
https://content.bartleby.com/qna-images/question/e912b8fc-dc8c-4789-829c-039b4c580bbb/fb9cf171-e5fd-4e8c-bd86-afc83b82d0da/gl1idi.png

1 How Many Moles Of Hydrogen H2 Are Needed To React With 2 6 Moles
https://us-static.z-dn.net/files/d78/e0f7a35e2ccde84c4b4eeec440788acf.png
The moles of N 2 and H 2 present originally were respectively Question A mixture of N 2 and H 2 is caused to react in a closed container to form NH 3 The reaction ceases before either reactant has been totally consumed At this stage 2 0 moles each of N 2 H 2 and NH 3 are present How many moles of N2 and H2 were present originally This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer Question A mixture of N2 g and H2 g reacts in a closed container to form ammonia NH3 g
How many moles of N2 and H2 were present originally B Nitrogen N2 and hydrogen H2 react to form ammonia NH3 Consider the mixture of N2 and H2 shown in the accompanying diagram The blue spheres represent N and the white ones represent H What is the limiting reactant in this case There are 2 steps to solve this one Expert verified Step 1 Write the balanced chemical equation 4NH g 7O g 4NO g 6H O l Step 2 Convert mass of NH moles of NH moles of NO 100 g NH 1mol NH3 17 03g NH3 4mol NO2 4mol NH3 5 872 mol NH 3 significant figures 1 guard digit Step 3 Use the Ideal Gas Law to calculate the volume of NO
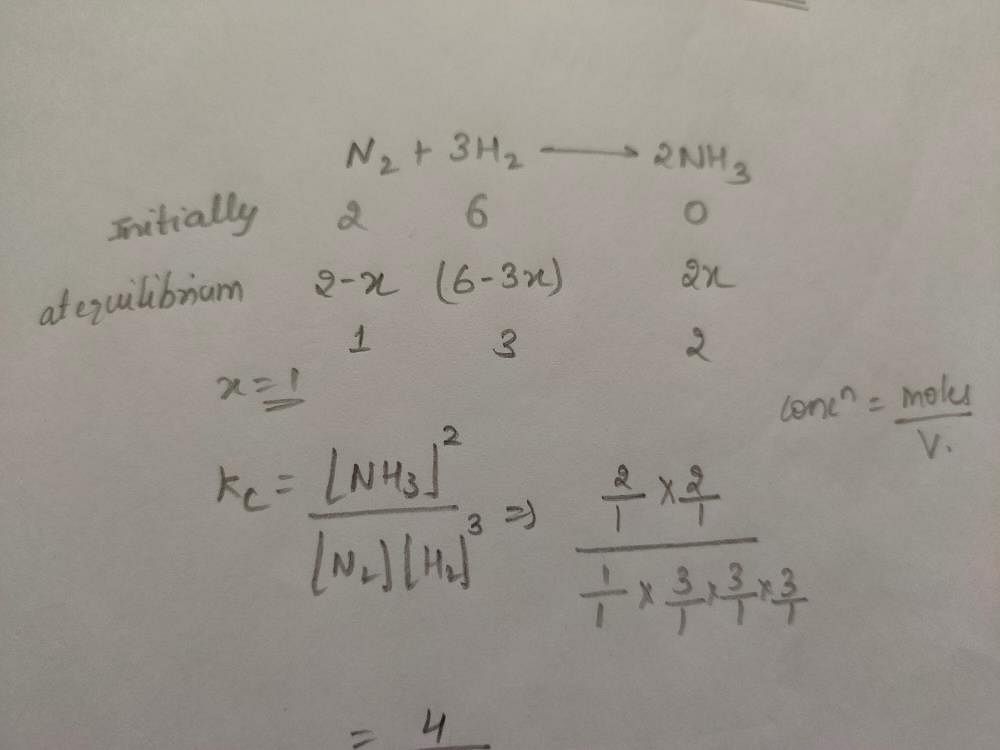
2 Moles Of N2 Is Mixed With 6 Moles Of H2 In A Closed Vessel Of One
https://edurev.gumlet.io/ApplicationImages/Temp/5517469_2d801615-3846-4d97-a4e5-e32c6b367c66_lg.png

N2 3H2 2NH3 1 Mole Of N2 And 4 Moles Of H2 Are Taken In 15 L Flask At
https://i.ytimg.com/vi/DLQpbtwnF-I/maxresdefault.jpg
How Many Moles Of N2 And H2 Were Present Originally - How many moles of O are required to form 5 00 moles of H O Solution 5 00 mol H O 1 mol O 2 mol H O 2 50 mol O If the question had been stated in terms of grams you would have had to convert grams of H O to moles of H O then moles of H O to moles of O as above and finally moles of O to grams of O