Formulas For Ionic Compounds Worksheet Answers Naming Chemical Compounds Answers Name the following ionic compounds 1 NaBr sodium bromide 2 CaO calcium oxide 3 Li 2 S lithium sulfide 4 MgBr 2 magnesium bromide 5 Be OH 2 beryllium hydroxide Write the formulas for the following ionic compounds 6 potassium iodide KI 7 magnesium oxide MgO 8 aluminum chloride AlCl 3
Find the formula for ionic compounds Naming ions and ionic compounds Science Chemistry library Atoms compounds and ions Names and formulas of ionic compounds Naming ionic compounds Google Classroom You might need Periodic table What is the systematic name of the following compound Al 4 C 3 Choose 1 answer Silver carbonate A Write the correct formula for each compound named below Show the ions from which it is formed 1 sodium chloride Na 1 Cl 1 NaCl Binary Ionic Formula Practice Name 1 sodium chloride Na 1 Cl 1 NaCl 2 lithium bromide Li 1 Br 1 LiBr 3 magnesium flouride Mg 2 F 1 MgF2
Formulas For Ionic Compounds Worksheet Answers
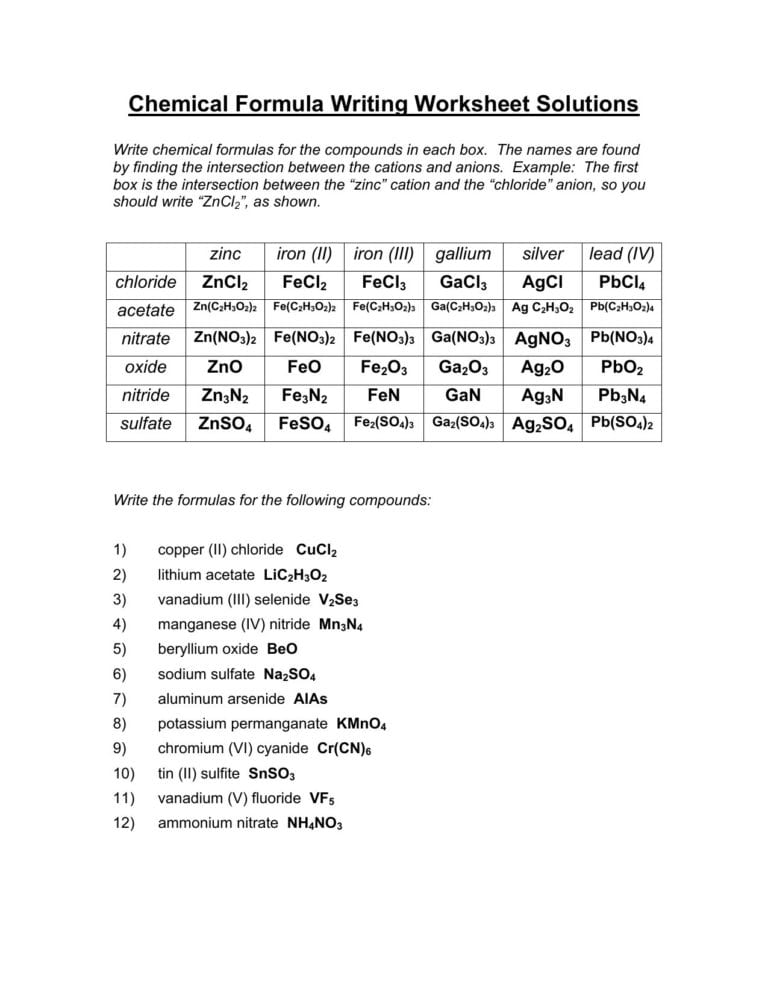
Formulas For Ionic Compounds Worksheet Answers
https://db-excel.com/wp-content/uploads/2019/09/ionic-compound-formula-writing-worksheet-answers-12-768x994-1.jpg
Molecular Compound Worksheet
http://1.bp.blogspot.com/_HSyZWoBHdDg/TMsPAJkvAvI/AAAAAAAAAlE/IkQIJRXDN2k/s1600/Naming+Ionic+Compounds+WS.JPG

Formulas And Nomenclature Binary Ionic Compounds Worksheet Answers
https://briefencounters.ca/wp-content/uploads/2018/11/formulas-and-nomenclature-binary-ionic-compounds-worksheet-answers-and-chemical-formulas-and-names-ionic-pounds-worksheet-awesome-of-formulas-and-nomenclature-binary-ionic-compounds-workshee.jpg
Nomenclature a collection of rules for naming things is important in science and in many other situations This module describes an approach that is used to name simple ionic and molecular compounds such as NaCl CaCO 3 and N 2 O 4 The simplest of these are binary compounds those containing only two elements but we will also consider how to name ionic compounds containing polyatomic ions Ionic Bonding and Writing Formulas Name KEY Part A Use the criss cross method to write the formulas produced from the listed ions Part B Write the names of the compounds formed in the above table Revised ST 7 29 13 LaBrake Vanden Bout 2013 Department of Chemistry University of Texas at Austin
This worksheet presents a widely used system of nomenclature for ionic compounds There are two types of metal cations with different naming conventions discussed separately fixed charge single charge cations Never use a Roman numeral variable charge multiple charge cations Always use a Roman numeral Cations with a single fixed charge Ionic Compounds Names and Formulas 1 Write the formulas for the following compounds a magnesium oxide n calcium chloride
More picture related to Formulas For Ionic Compounds Worksheet Answers

Binary Ionic Compounds Worksheet Answers Main Idea Db excel
https://db-excel.com/wp-content/uploads/2019/09/binary-ionic-compounds-worksheet-answers-main-idea-3.jpg

Writing And Naming Binary Ionic Compounds Worksheet Answers Worksheet
https://i2.wp.com/thesecularparent.com/wp-content/uploads/2020/04/writing-ionic-compounds-worksheet-answers.jpg

Ionic Compounds And Metals Worksheet Answers
https://i1.wp.com/briefencounters.ca/wp-content/uploads/2018/11/naming-ionic-compounds-worksheet-one-as-well-as-how-to-write-ionic-pounds-worksheet-new-naming-ionic-pounds-and-of-naming-ionic-compounds-worksheet-one.jpg?fit=809%2C1024&ssl=1
Write the formulas for the following compounds magnesium oxide MgO bl sodium fluoride NaF cl aluminum nitride AlN d potassium sulfide e lithium iodide K2S LiI fl calcium bromide CaBr2 g beryllium oxide 11 h nickel chloride fl magnesium nitride BeO NiCl2 Mg3N2 Al2S3 j aluminum sulfide Write the names for the following compounds School subject Chemistry 1061818 Main content Ionic compounds 1985115 Naming and writing formulas for ionic compounds Other contents Naming writing formulas
All ionic compounds follow this rule the charge from the positive ions must be equal to the charge from the negative ions In a compound of aluminum ion Al3 and nitrate ion NO3 1 there must be three nitrate ions So the formula for this compound is Al NO3 3 Recall that nitrate is a polyatomic ion so this combination of atoms remains A binary ionic compound is a compound composed of a monatomic metal cation and a monatomic nonmetal anion The metal cation is named first followed by the nonmetal anion as illustrated in Figure 4 5 1 4 5 1 for the compound BaCl 2 The word ion is dropped from both parts Figure 4 5 1 4 5 1 Naming BaCl2 B a C l 2
Naming Ionic Compounds SliderBase Worksheet Template Tips And Reviews
http://www.sliderbase.com/images/referats/141b/(28).PNG
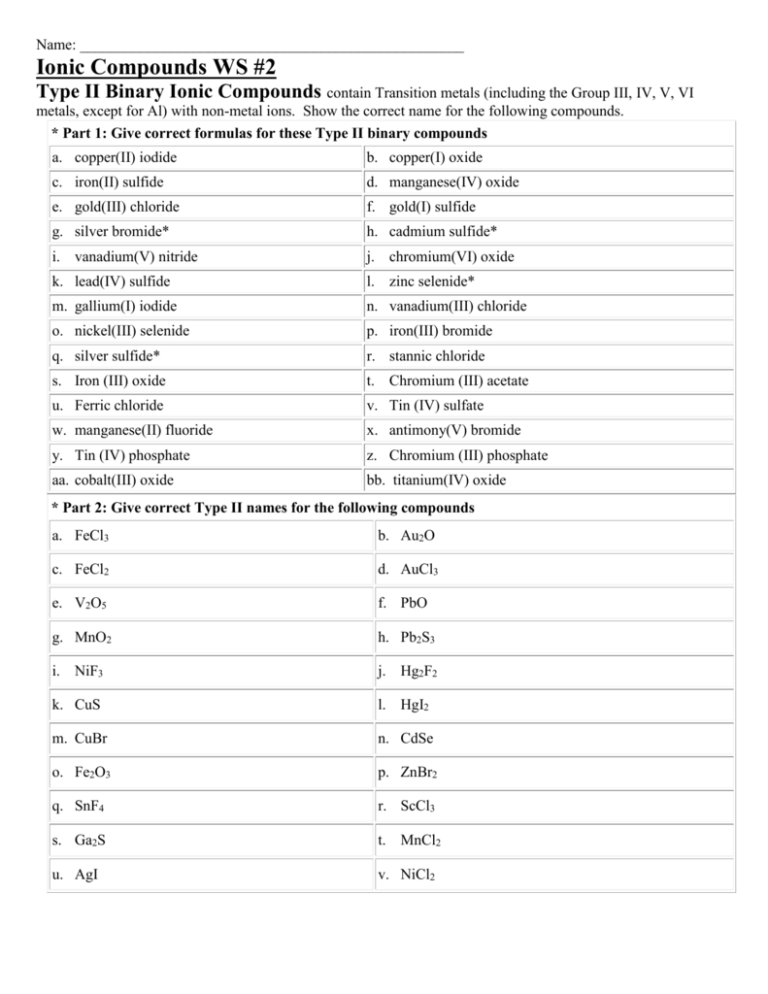
Ionic Compounds WS 2
https://s3.studylib.net/store/data/008468944_1-6f4c622c9bef12df1d98cbc164723546-768x994.png
Formulas For Ionic Compounds Worksheet Answers - This worksheet presents a widely used system of nomenclature for ionic compounds There are two types of metal cations with different naming conventions discussed separately fixed charge single charge cations Never use a Roman numeral variable charge multiple charge cations Always use a Roman numeral Cations with a single fixed charge
.PNG)