F2 H2o Balanced Equation How to Balance F2 H2O OF2 HF Fluorine gas Water MagnetsAndMotors Dr B s Other Channel 34 1K subscribers Subscribe Subscribed 14K views 3 years ago In this video we ll balance the
We will look how to balance them in redox method Balance F 2 H 2 O HF O 2 First find oxidation numbers of each element in the left side and right side of the equation Then determine which atoms oxidation numbers are changed when going to the right side Oxidation number of fluorine has been changed from 0 to 1 Balancing step by step using the algebraic method Let s balance this equation using the algebraic method First we set all coefficients to variables a b c d a F 2 b H 2 O c OF 2 d HF Now we write down algebraic equations to balance of each atom F a 2 c 2 d 1 H b 2 d 1 O b 1 c 1
F2 H2o Balanced Equation
F2 H2o Balanced Equation
https://i.ytimg.com/vi/Vu3VfyHqwts/maxresdefault.jpg

F2 H2o Lewis G sterimi Nas l Eodev
https://tr-static.eodev.com/files/dca/8b4c09929cae7a913268854c67b29ecb.png
Solved The Following Reaction Occurs In Basic Solution F2 Chegg
https://media.cheggcdn.com/study/f03/f035495d-e8a4-4993-98d5-475fea1aec9f/image
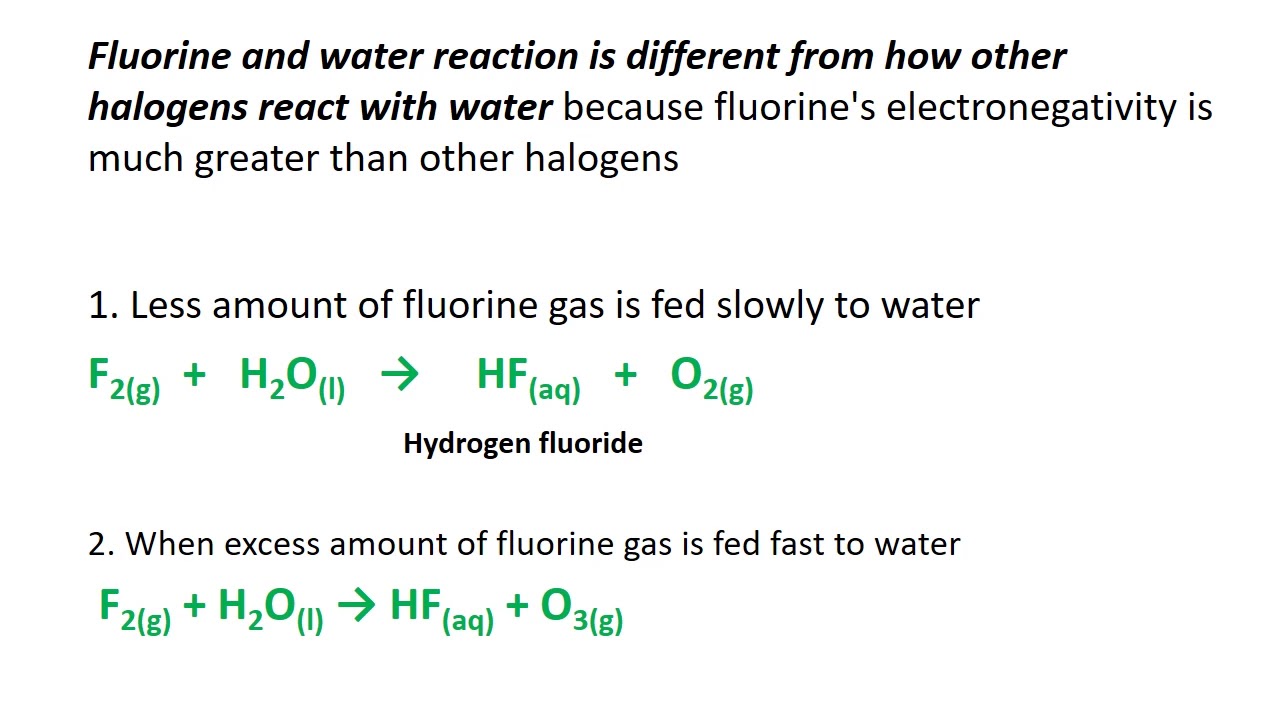
Error equation F2 H2O HF is an impossible reaction Please correct your reaction or click on one of the suggestions below F2 H2O HF O2 F2 H2O HF O3 Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents The limiting reagent row will be highlighted in pink The equation is separated into two half equations one for oxidation and one for reduction The equation is balanced by adjusting coefficients and adding H 2 O H and e in this order Balance the atoms in the equation apart from O and H To balance the Oxygen atoms add the appropriate number of water H 2 O molecules to the other side
If a fractional coefficient has been used multiply both sides of the equation by the denominator to obtain whole numbers for the coefficients Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced Example 7 4 1 7 4 1 Combustion of Heptane The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter
More picture related to F2 H2o Balanced Equation
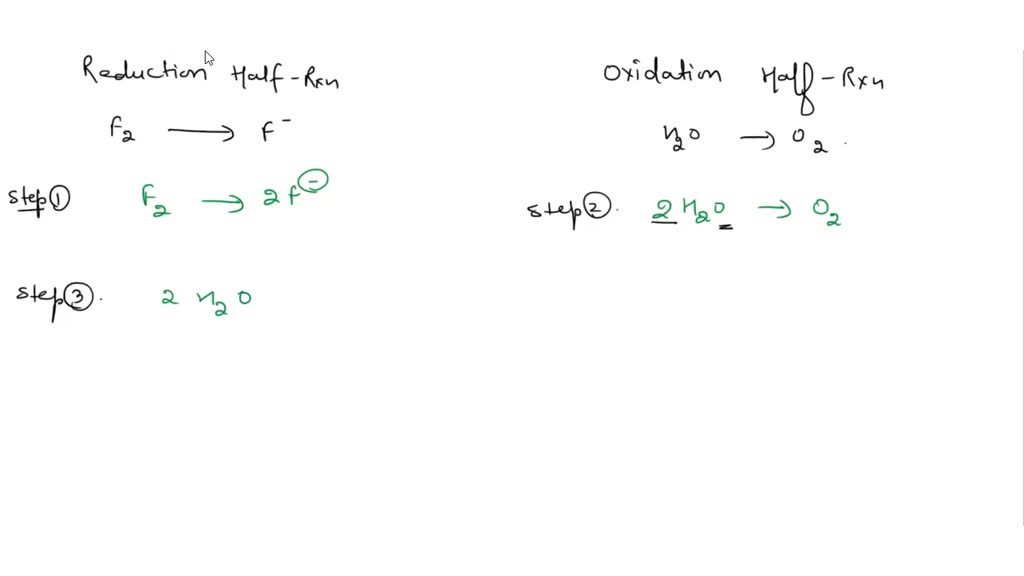
SOLVED The Following Reaction Occurs In Basic Solution F2 H2O
https://cdn.numerade.com/ask_previews/bf4758c9-034e-447a-94cc-592f13507d3d_large.jpg
How To Write The Equation For FeF2 H2O YouTube
https://i.ytimg.com/vi/PXVRnBBaLCQ/maxresdefault.jpg
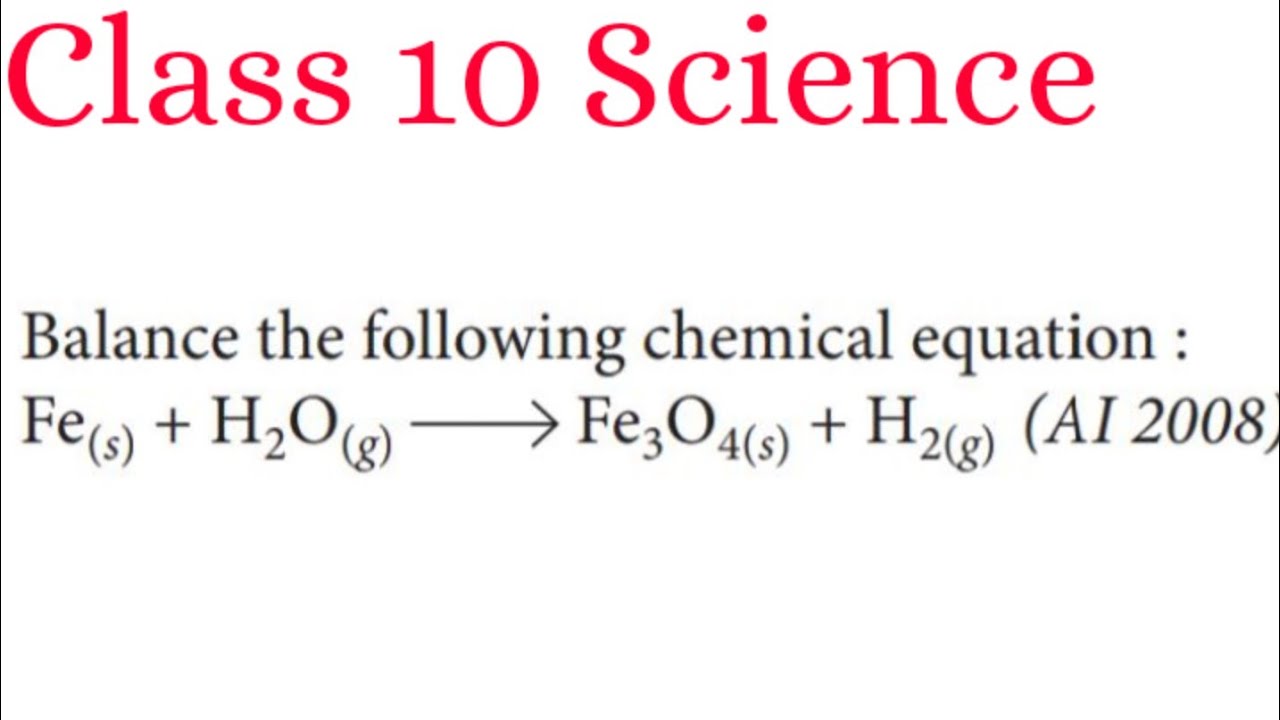
Balance The Chemical Equation Fe s H2O g Fe3O4 s H2 g
https://i.ytimg.com/vi/zLeJbLlZCnU/maxresdefault.jpg
Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced Example 3 2 1 Combustion of Heptane Balance the chemical equation for the combustion of Heptane C 7 H 16 C 7 H 16 l O 2 g CO 2 g H 2 O g The equation is balanced by changing the scalar numbers that precede each part of the equation Chemistry Science Anatomy Physiology Astronomy What is the balanced equation of C6H6 O2 CO2 H2O How can I balance this chemical equation Pb NO3 2 K2CrO4 PbCrO4 KNO3 How do you balance Al F 2 AlF 3
The following reaction occurs in basic solution F2 H2O O2 F When the equation is balanced the sum of the coefficients is This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts See Answer Chemistry Chemistry questions and answers The following reaction occurs in basic solution F2 H2O O2 F When the equation is balanced the sum of the coefficients is Select one a none of theseb 10c 11d 13e 12 This problem has been solved You ll get a detailed solution from a subject matter expert that helps you learn core concepts

The Advantages Of Using Chlorine Over Fluorine GroundWaterGovernance
https://groungims.groundwatergovernance.org/chlorine_and_fluorine_reaction.jpg
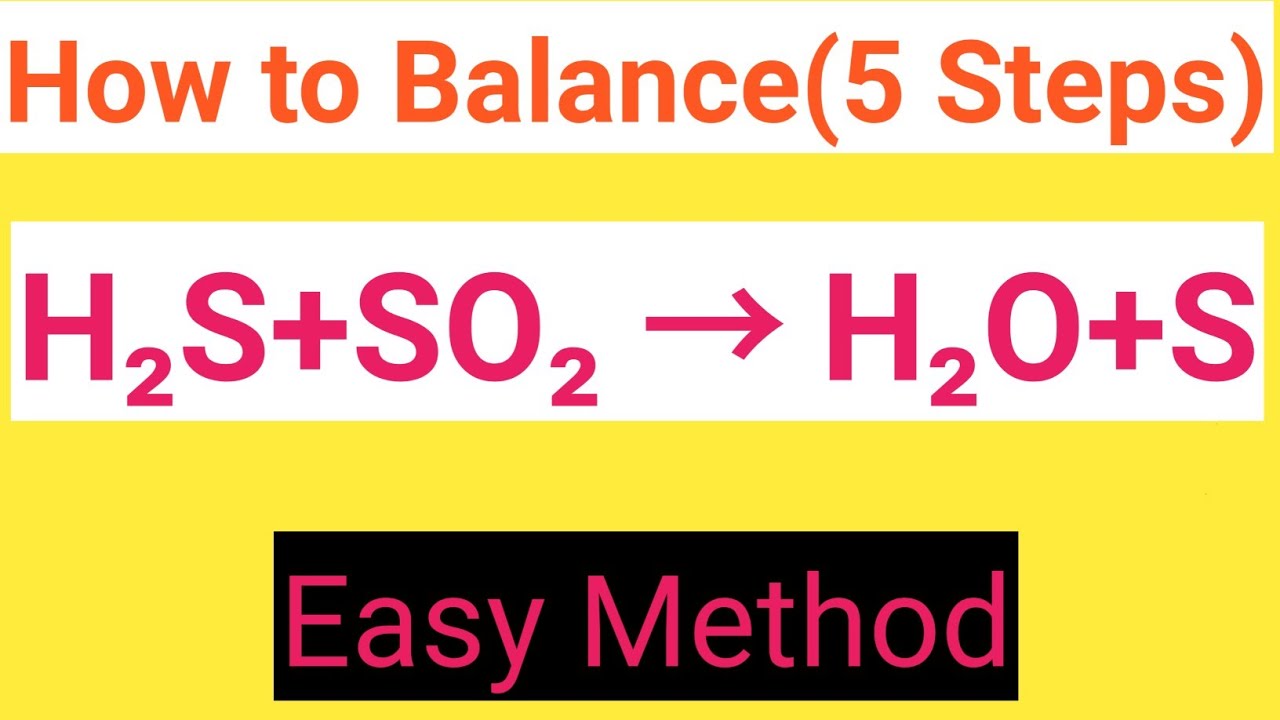
H2S SO2 H2O S Balanced Equation Hydrogen Sulphide Sulphur Dioxide
https://i.ytimg.com/vi/90Mu4PRXB7I/maxresdefault.jpg
F2 H2o Balanced Equation - F2 fluorine solid H2O water solid O2 oxygen solid HF hydrogen fluoride solid Temperature temperature Other Condition excess chlorine
