Experiment 25 Report Sheet Part A The 200 mm test tube also contained some water besides the metal that was subsequently added to the calorimeter in Part A Considering a higher specific heat for water will the temperature change in the calorimeter be higher lower or unaffected by this technique error Explain Part A
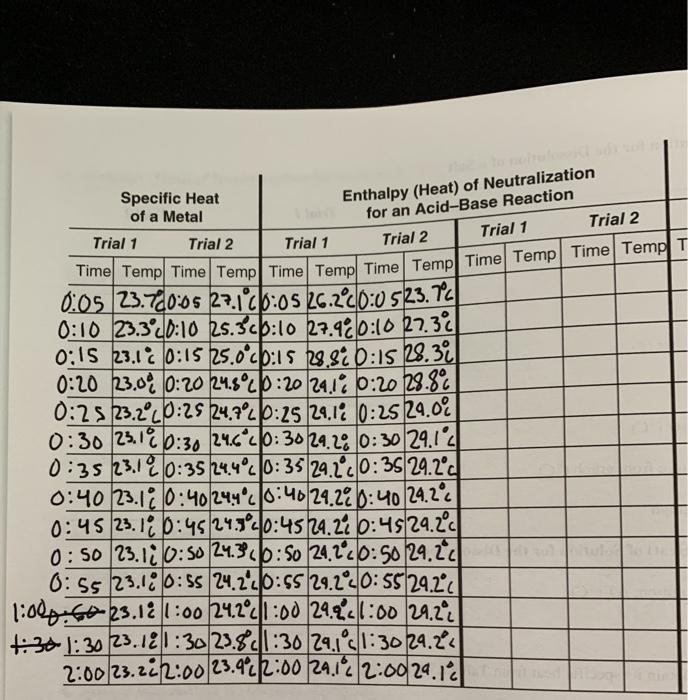
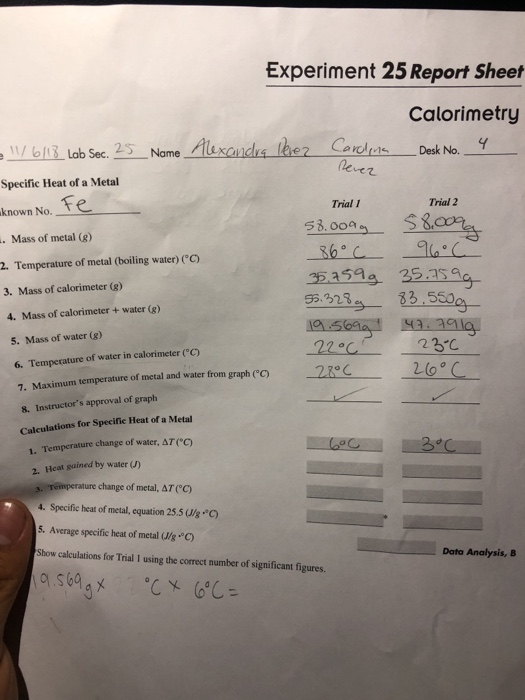
Question Experiment 25 Report Sheet Calorimetry Date Lab Sec Name Desk No A Specific Heat of a Metal Unknown No Trial 1 Trial 2 1 Mass of metal 8 2 Temperature of metal boiling water C 99 10 c 20 477g 14 654g 94 00 C 60 299g 60 5919 79 4459 44 79 7739 3 Mass of calorimeter g 4 Mass of calorimeter water 8 5 Explain Part A In measuring the specific heat of a metal Josh used the highest measured temperature for calculating themetal s specific heat rather then the extrapolated temperature Will this decision result in a higher or lower specific heat value for the metal Explain Part B The enthalpy of neutralization for error Explain
Experiment 25 Report Sheet
Experiment 25 Report Sheet
https://media.cheggcdn.com/media/d99/d99c13c7-da8f-4594-93d0-9b9193f79857/image

Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Chegg
https://d2vlcm61l7u1fs.cloudfront.net/media/ae0/ae06194c-0e1b-4306-898c-f98c16c48bdb/php5hdyA1.png
Solved Experiment 25 Report Sheet Calorimetry Desk No A Chegg
http://d2vlcm61l7u1fs.cloudfront.net/media/41e/41e70901-e42f-4382-8d5e-bbb2d2b15d88/image
Experiment 25 Report Sheet Calorimetry Date Lab Sec Name Desk No A Specific Heat of a Metal Unknown No A 1 Mass of metal 8 2 Temperature of metal boiling water C Trial 1 23 497 10 2 37608 51 403 Trial 2 22 324 100 2 37 623 57 514 3 Mass of calorimeter 8 4 Mass of calorimeter water 8 5 Mass of water 8 6 Experiment 25 Report Sheet Calorimetry Lab Sec Name Date A Specific Heat of a Metal Unknown No Desk No Trial 1 Trial 2 15002 22 791 1 Mass of metal g 2 Temperature of metal boiling water C 3 Mass of calorimeter g 4 Mass of calorimeter water g 5
Download Experiment Lab 25 Calorimetry and more Chemistry Lab Reports in PDF only on Docsity Experiment 25 Calorimetry Enthalpies and Specific Heats Pre Lab Hints 1 Explain how the temperature of the metal and the water bath become equalized and how that final equalized temperature is measured 2 Study with Quizlet and memorize flashcards containing terms like Part A 1 The 200 mm test tube also contained some water besides the metal that was subsequently added to the calorimeter in Part A 4 Considering a higher specific heat for water will the temperature change in the calorimeter be higher lower or unaffected by this technique error Explain Part B The enthalpy of
More picture related to Experiment 25 Report Sheet
Solved Experiment 25 Report Sheet Calorimetry 613 Lob Sec Chegg
https://media.cheggcdn.com/media/4d8/4d8f24da-a96d-4d72-9c24-c12a15bdd45b/image.png
Solved Experiment 25 Report Sheet Calorimetry Date Lab Sec Chegg
https://media.cheggcdn.com/study/f3c/f3c735f8-0d70-4bc2-a418-b4273d30a164/image

Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Chegg
https://media.cheggcdn.com/media/639/639ae0d5-e37e-4ccd-87bb-6fecac0607f6/image.png
Lab report experiment 25 calorimetry lab amber rampersaud hans bernard isabella perez general chemistry d02 professor richard perry 21 october 2020 Skip to document Experiment 25 Calorimetry On Campus Lab Amber Rampersaud Hans Bernard Isabella Perez General Chemistry I D02 Professor Richard H Perry 21 October 2020 Which of the following is the correct order to perform Part A Specific Heat of a Metal step 3 Take mass of your metal Add it into a large test tube Which of the following is the correct order to perform Part A Specific Heat of a Metal step 4 Heat metal in water bath to boiling then heat 10 more minutes
Calorimetry I Introduction The objective of this lab was to determine the specific heat of a metal determine the enthalpy of neutralization for a strong acid strong bas and to determine the enthalpy of solution for the dissolution of a salt II Experimental Section Report sheet attached III Experiment 25 Report Sheet Part C Determination of pKa of Acetic Acid Volume at equivalence point o Solution 2 18 95mL 1 00mL 17 95mL of NaOH o Solution 3 32 67mL 14 90mL 17 77mLof NaOH Volume at one half equivalence point o Solution 2 10 15mL 1 00mL 9 15mLof NaOH o Solution 3 23 75mL 14 90mL 8 85mL NaOH pKa Determined Graphicall
Solved Experiment 25 Report Sheet Calorimetry Date Lab Sec Chegg
https://media.cheggcdn.com/study/59b/59b89dbb-dc9a-4266-a2a7-49a3d6ce4f1a/image

EXPERIMENT 25 REPORT SHEET Determination Of The Dissociation Constant
https://img.homeworklib.com/questions/bdd1dc30-78e5-11ea-ac37-2154599a3dda.png?x-oss-process=image/resize,w_560
Experiment 25 Report Sheet - Study with Quizlet and memorize flashcards containing terms like Part A 1 The 200 mm test tube also contained some water besides the metal that was subsequently added to the calorimeter in Part A 4 Considering a higher specific heat for water will the temperature change in the calorimeter be higher lower or unaffected by this technique error Explain Part B The enthalpy of