Examine The Following Unbalanced Chemical Equation Figure 4 2 4 4 2 4 The Relationships among Moles Masses and Formula Units of Compounds in the Balanced Chemical Reaction for the Ammonium Dichromate Volcano Chemical equation N H 4 2 C r 2 O 7 dissociates into C r 2 O 3 N 2 and H 2 O Conversions are given between moles mass and molecules
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button The balanced equation will appear above Use uppercase for the first character in the element and lowercase for the second character Examples Fe Au Co Br C O N F Ionic charges are not yet supported and will be ignored 4 1 4 2 2 4 4 4 yes O 2 2 4 1 2 2 1 4 4 4 yes A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection Consider as an example the decomposition of water to yield molecular hydrogen and oxygen
Examine The Following Unbalanced Chemical Equation
Examine The Following Unbalanced Chemical Equation
https://media.cheggcdn.com/study/2d6/2d69d5f9-67bd-4df0-a104-3583a4a569b3/image

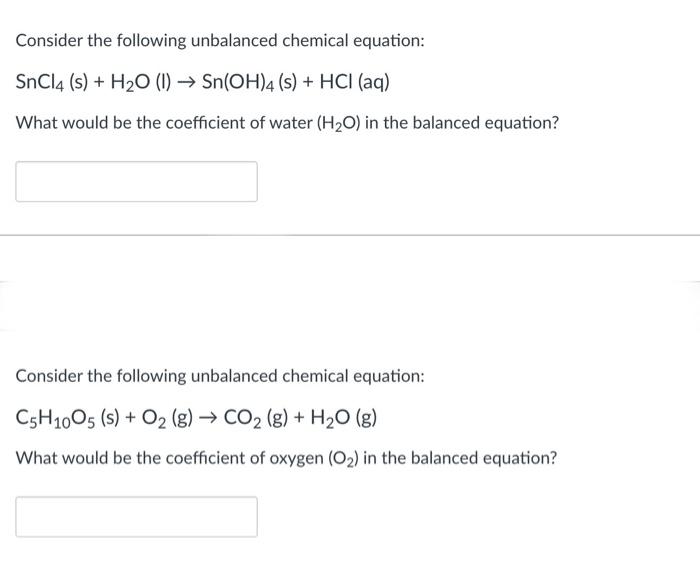
Consider The Following Unbalanced Chemical Equation LiOH s CO2 g
https://us-static.z-dn.net/files/dab/83caea61e80c6b1c27cb0003c75c79c7.png
Solved Consider The Following Unbalanced Chemical Equation Chegg
https://media.cheggcdn.com/media/d27/d27cc90d-02b5-49de-ae7e-01195ce4b64f/phppzRrsf
Chemical Equations and the Law of Conservation of Matter In the previous section the reaction between hydrogen gas and oxygen gas to produce water in the gaseous phase was shown as a chemical equation H 2 g O 2 g H 2 O g At the molecular level the reaction would look something like this Notice that there are two oxygen atoms on the left hand side of the equation and only Balance the following chemical equation Mg OH 2 HCl MgCl 2 H 2 O Note All reactants and products require a coefficient of at least one Stuck Review related articles videos or use a hint
CO2 H2O C6H12O6 O2 The first step to balancing chemical equations is to focus on elements that only appear once on each side of the equation Here both carbon and hydrogen fit this requirement So we will start with carbon There is only one atom of carbon on the left hand side but six on the right hand side On this side we have one two three four five six oxygens In order to balance it we re going to have six oxygens on the reactant side We need three of these molecules So another one and another one right over there And now we re all balanced We have two carbon atoms on both sides Carbons carbons
More picture related to Examine The Following Unbalanced Chemical Equation
50 Examples Of Unbalanced Chemical Equations With Answers
https://i2.wp.com/d2vlcm61l7u1fs.cloudfront.net/media/cef/cef6dc19-1a0c-416c-83f5-ebd1194bc26c/image
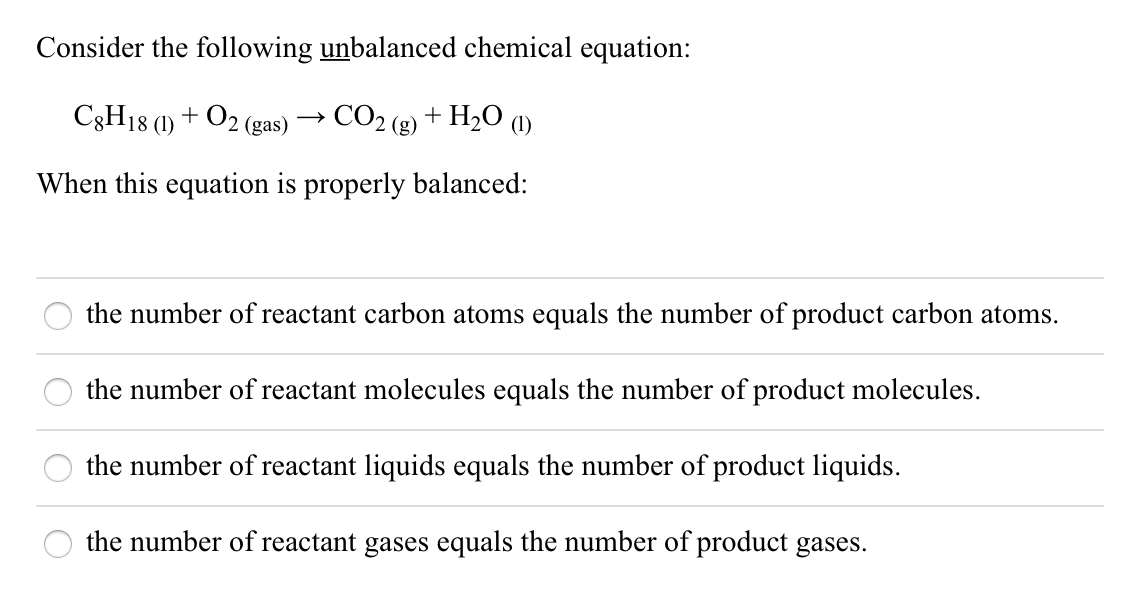
Solved Consider The Following Unbalanced Chemical Equation Chegg
https://media.cheggcdn.com/media/d62/d62492d2-d74e-4e22-a5a3-5c975a5da404/php3CB6kM.png

ANSWERED Use The Following Unbalanced Chemical Equ Physical
https://media.kunduz.com/media/sug-question/raw/54505578-1657539632.1317263.jpeg?h=512
Examine the following unbalanced chemical equation CO2 C CO Which of the following is the correctly balanced form of this equation A CO2 C2 CO B CO2 C 2CO C 2CO2 C CO D CO2 C C2O The chemical equation described in section 4 1 is balanced meaning that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides This is a requirement the equation must satisfy to be consistent with the law of conservation of matter It may be confirmed by simply summing the numbers of
The balanced equation for CO2 C CO is C CO2 2CO The correct answer is option C CO2 C 2CO This is because the equation must satisfy the Law of Conservation of Mass meaning the number of atoms on each side of the equation must be equal The equation as given is unbalanced since there is only one carbon atom on the left hand side and two on the right hand side To balance the Instructions on balancing chemical equations Enter an equation of a chemical reaction and click Balance The answer will appear below Always use the upper case for the first character in the element name and the lower case for the second character Examples Fe Au Co Br C O N F Compare Co cobalt and CO carbon monoxide

50 Examples Of Unbalanced Chemical Equations Brainly in
https://hi-static.z-dn.net/files/d53/b4155420413703af7f5d366284029749.jpg
50 Examples Of Unbalanced Chemical Equations With Answers
https://i2.wp.com/lh6.googleusercontent.com/proxy/nVgxwXt2_vcSQJCtHlbli1H8jwvle29ZgUAp3BBEVbdJVg3gTkiw-msIGj0za9b6Y-S5dkhfKhmnbpzsPUuibGnbm-PZgCI4AlKLowaD7aDdoKx08XqbmgJGNy8vs0VBD82CjKSzhTazILwiPf_48wxWqdTFTN9ILt8=w1200-h630-p-k-no-nu
Examine The Following Unbalanced Chemical Equation - How do you know if a chemical equation is balanced What can you change to balance an equation Play a game to test your ideas